Research



Biomolecular solid-state NMR
Our research targets key questions about structure, dynamics and interactions in complex biomolecular systems by developing state-of-the-art solid-state nuclear magnetic resonance (ssNMR) methodology.
ssNMR is a powerful atomic-scale characterization technique applicable to systems that cannot be investigated by neither solution NMR nor X-ray crystallography, ranging from non-crystalline or poorly-crystalline assemblies to protein aggregates or fibrils. Our objective is to provide unique insight into structure and function for the understanding of central biochemical and genetic processes which cannot be accessed at atomic resolution by any other experimental technique. This knowledge is key to control these complex systems, modify their behaviour, and design improved ligands, that could be lead compounds for the development of new drugs.
Our team is highly interdisciplinary, and connects students and post-docs performing research spanning a wide range of disciplines, ranging from molecular biology, as of importance in protein over-expression and purification, via physical chemistry necessary to acquire and interpret sophisticated NMR experiments, to structural biology.
Recently, we have pioneered a new approach based on small-volume rotors spinning at the magic angle under ultra-fast (>60 kHz) rates. We have shown that in combination with 100% NH re-protonation in a perdeuterated background and high magnetic fields, this enables the acquisition of resolved 1H resonances, yielding rapid “fingerprint” spectra, and opening up a sensitive way to the detection of a range of structurally important parameters. These developments speed up by almost two orders of magnitude backbone resonance assignment, structure determination and dynamic descriptions of microcrystalline proteins of considerable size, clearly opening the way to more complex biological solids, of higher molecular weight or available in very limited amounts. Our current activity aims to capitalize on these new concepts, and exploiting the most sophisticated instrumental technology that is now uniquely available in our laboratory, to develop improved NMR methodology and new biochemical strategies to push forward the limits of the applicability of this technique to complex, dynamical, non-crystalline macromolecular assemblies, and to improve significantly the structural information that can be obtained from these systems.
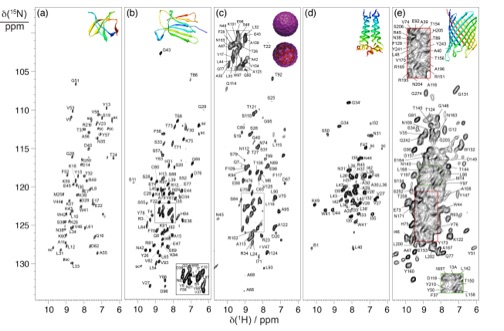
Our research in this field is articulated along a series of concerted research themes, each of which provides an exemplary structural and functional challenge to the ssNMR approach:
-
(i)Protein-protein interactions in the bacterial replisome (in collaboration with Prof. N. E. Dixon, University of Wollongong, Australia)
-
(ii)Molecular basis of replication in Measles virus (in collaboration with M. Blackledge, M. Jensen and R. Ruigrock, IBS Grenoble)
-
(iii)Towards high resolution ssNMR characterization of pathologic protein aggregates (in collaboration with S. Ricagno and M. Bolognesi, University of Milan, and V. Bellotti, University of Pavia, Italy and UC London, UK)
-
(iv)Structure and dynamics of metalloproteins (in collaboration with R. PIerattelli, I. C. Felli, G. Parigi and C. Luchinat, University of Florence)
-
(v)Methods for structure and dynamics of membrane proteins (in collaboration with R. G. Griffin, MIT, J. J. Chou, Harvard, and H. Oschkinat, FMP Berlin)
Paramagnetic solid-state NMR
Metal ions are present at the active sites of many catalytic processes that are at the core of modern chemistry, are the key constituents of many new versatile materials, and play an important role in a large variety of biochemical and cellular processes. As such, they have a tremendous impact on many fields within industry, energy, environment and life sciences.
The aim of our research in this area is to develop solid-state NMR into a robust tool for the structure characterization of complex samples containing paramagnetic metal centres, such as battery materials, supported catalysts or metalloproteins.
The field has experience a real revolution in the recent years. Combined progress in sample preparation, probe technology, and rf irradiation schemes has opened the way to the first examples of high-resolution NMR in paramagnetic solids, and to the use of paramagnetic effects as a powerful source of restraints for structure determination of small and large metal-containing systems.
Despite this recent progress, however, a number of issues remain to be addressed before paramagnetic NMR can become a routine tool for the targets of increasing complexity that keep being profiled by modern research in chemistry and biology. With our research, we want to address the key remaining bottlenecks to NMR of paramagnetic solids, as concerns the steps of both acquisition and interpretation of the NMR spectra, and extend the fields of application, attacking relevant chemical and biological problems, with novel techniques to determine structure (e.g., of insoluble proteins and disordered battery electrode materials), dynamics and reactivity around metal centres, and exploring interactions between, e.g., biomolecules, catalytic centres and supports.
Recent examples include the characterization of a series of novel cathode materials (in collaboration with C. P. Grey, University of Cambridge), of the local environments of dilute activator ions in the solid-state lighting phosphor (in collaboration with B. Chmelka, University of California at Santa Barbara), and the full structural and dynamical determination of a microcrystalline copper metalloenzyme (in collaboration with I. Bertini, R. Pierattelli and I. C. Felli, University of Florence).