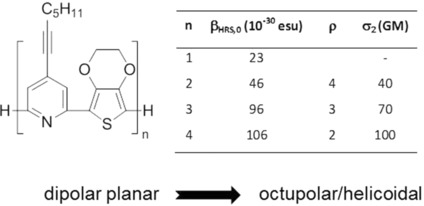
https://doi.org/10.1002/cphc.201601057
F. Chevallier, M. Charlot, F. Mongin, B. Champagne, E. Franz, K. Clays, M. Blanchard-Desce, ChemPhysChem 2016, 17, 4090-4101.

https://doi.org/10.1002/cphc.201601057
F. Chevallier, M. Charlot, F. Mongin, B. Champagne, E. Franz, K. Clays, M. Blanchard-Desce, ChemPhysChem 2016, 17, 4090-4101.
https://doi.org/10.1039/C6NJ02018F
P. Srinivas, S. Prabhakar, F. Chevallier, E. Nassar, W. Erb, V. Dorcet, V. Jouikov, P. Radha Krishna, F. Mongin, New J. Chem. 2016, 40, 9441-9447.
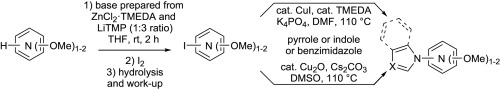
https://doi.org/10.1016/j.tet.2016.08.056
M. Hedidi, W. Erb, G. Bentabed-Ababsa, F. Chevallier, L. Picot, V. Thiéry, S. Bach, S. Ruchaud, T. Roisnel, V. Dorcet, F. Mongin, Tetrahedron 2016, 72, 6467-6476.
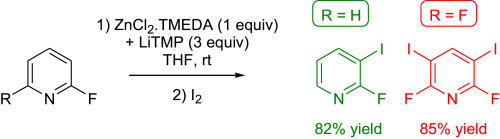
https://doi.org/10.1016/j.tet.2016.03.022
M. Hedidi, G. Bentabed-Ababsa, A. Derdour, Y. S. Halauko, O. A. Ivashkevich, V. E. Matulis, F. Chevallier, T. Roisnel, V. Dorcet, F. Mongin, Tetrahedron 2016, 72, 2196-2205.

https://doi.org/10.1002/9781118754887.ch27
F. Chevallier, F. Mongin, R. Takita, M. Uchiyama, in Arene Chemistry: Reaction Mechanisms and Methods for Aromatic Compounds (Ed.: J. Mortier), John Wiley & Sons, Hoboken, 2016, pp. 777-812.
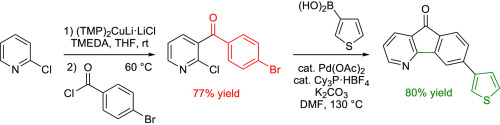
https://doi.org/10.1016/j.tet.2015.12.050
N. Marquise, F. Chevallier, E. Nassar, M. Frédérich, A. Ledoux, Y. S. Halauko, O. A. Ivashkevich, V. E. Matulis, T. Roisnel, V. Dorcet, F. Mongin, Tetrahedron 2016, 72, 825-836.
https://doi.org/10.1055/s-0035-1560360
D. Tilly, F. Chevallier, F. Mongin, Synthesis 2016, 48, 184-199.
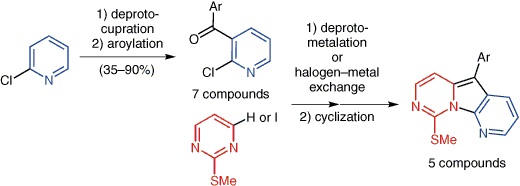
https://doi.org/10.1055/s-0035-1560496
N. Marquise, T. T. Nguyen, F. Chevallier, L. Picot, V. Thiéry, O. Lozach, S. Bach, S. Ruchaud, F. Mongin, Synlett 2015, 26, 2811-2816.
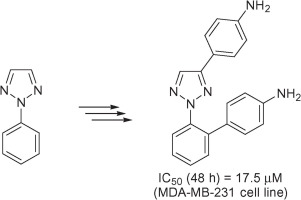
https://doi.org/10.1016/j.bmc.2015.08.031
E. Nagaradja, G. Bentabed-Ababsa, M. Scalabrini, F. Chevallier, S. Phillipot, S. Fontanay, R. E. Duval, Y. S. Halauko, O. A. Ivashkevich, V. E. Matulis, T. Roisnel, F. Mongin, Bioorg. Med. Chem. 2015, 23, 6355-6363.
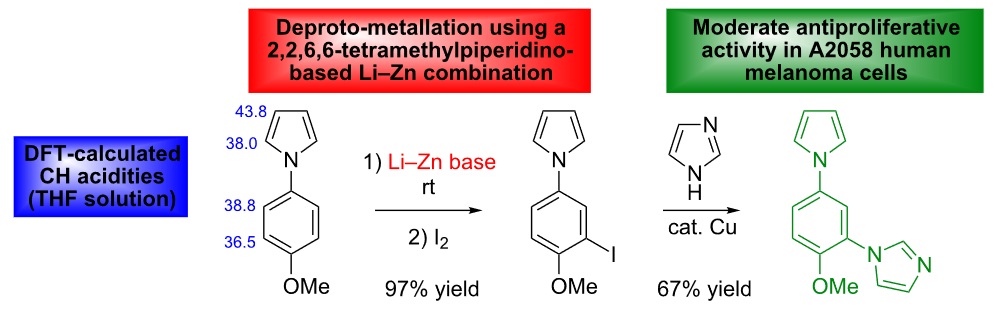
https://doi.org/10.3762/bjoc.11.160
M. Y. Ameur Messaoud, G. Bentabed-Ababsa, M. Hedidi, A. Derdour, F. Chevallier, Y. S. Halauko, O. A. Ivashkevich, V. E. Matulis, L. Picot, V. Thiéry, T. Roisnel, V. Dorcet, F. Mongin, Beilstein J. Org. Chem. 2015, 11, 1475-1485.