https://doi.org/10.1016/j.tet.2012.02.019
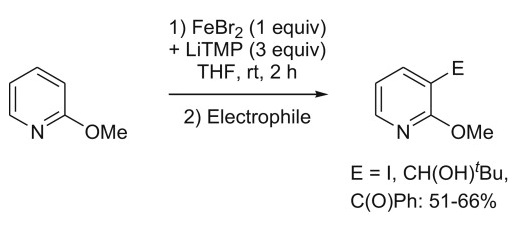
E. Nagaradja, F. Chevallier, T. Roisnel, V. Jouikov, F. Mongin, Tetrahedron 2012, 68, 3063-3073.

https://doi.org/10.1016/j.tet.2012.02.019
E. Nagaradja, F. Chevallier, T. Roisnel, V. Jouikov, F. Mongin, Tetrahedron 2012, 68, 3063-3073.

https://doi.org/10.1002/ejoc.201101490
D. Catel, F. Chevallier, F. Mongin, P. C. Gros, Eur. J. Org. Chem. 2012, 53-57.
https://doi.org/10.1002/chem.201101993
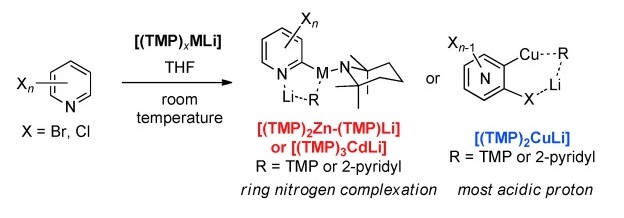
K. Snégaroff, T. T. Nguyen, N. Marquise, Y. S. Halauko, P. J. Harford, T. Roisnel, V. E. Matulis, O. A. Ivashkevich, F. Chevallier, A. E. H. Wheatley, P. C. Gros, F. Mongin, Chem. Eur. J. 2011, 17, 13284-13297.
https://doi.org/10.1002/chem.201100990
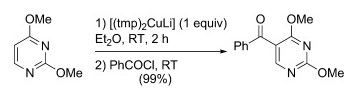
T. T. Nguyen, N. Marquise, F. Chevallier, F. Mongin, Chem. Eur. J. 2011, 17, 10405-10416.
https://doi.org/10.1002/ejoc.201100302
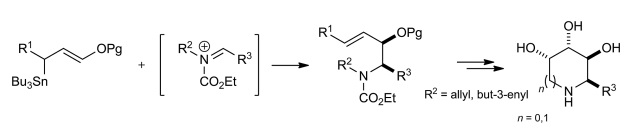
F. Chevallier, A. Lumbroso, I. Beaudet, E. Le Grognec, L. Toupet, J.-P. Quintard, Eur. J. Org. Chem. 2011, 4133-4144.
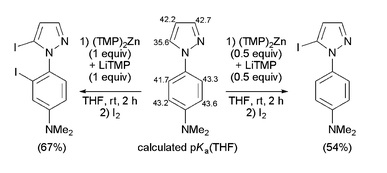
https://doi.org/10.1039/c1ob05267e
F. Chevallier, Y. S. Halauko, C. Pecceu, I. F. Nassar, T. U. Dam, T. Roisnel, V. E. Matulis, O. A. Ivashkevich, F. Mongin, Org. Biomol. Chem. 2011, 9, 4671-4684.
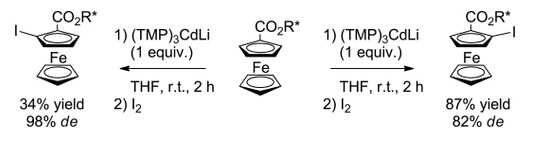
https://doi.org/10.1002/ejoc.201100264
A. Sreeshailam, G. Dayaker, F. Chevallier, T. Roisnel, P. Radha Krishna, F. Mongin, Eur. J. Org. Chem. 2011, 3715-3718.
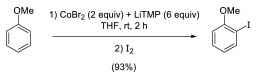
https://doi.org/10.1016/j.tet.2010.09.053
G. Dayaker, F. Chevallier, P. C. Gros, F. Mongin, Tetrahedron 2010, 66, 8904-8910.
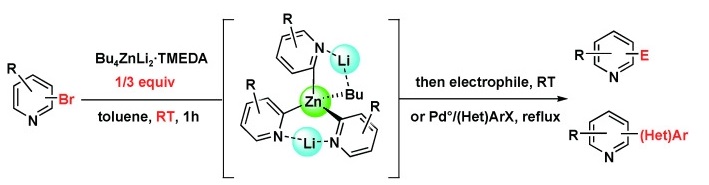
https://doi.org/10.1002/chem.201001664
N. T. T. Chau, M. Meyer, S. Komagawa, F. Chevallier, Y. Fort, M. Uchiyama, F. Mongin, P. C. Gros, Chem. Eur. J. 2010, 16, 12425-12433.
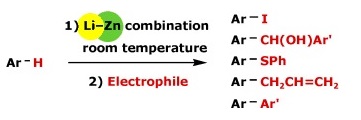
https://doi.org/10.1002/chem.201000543
K. Snégaroff, S. Komagawa, F. Chevallier, P. C. Gros, S. Golhen, T. Roisnel, M. Uchiyama, F. Mongin, Chem. Eur. J. 2010, 16, 8191-8201.