After a MSc degree at INSA Rouen, I moved to University of Strasbourg for a PhD supported by a MENRT fellowship. My doctoral work was focused on the light driven production of hydrogen and on the synthesis of novel fluorescent dyes. Then I worked on STM analyses of photoresponsive self-assembled monolayers as a JSPS fellow at Kyoto University, on redox-active molecular layers for electronic devices at Paris Diderot University and binuclear phthalocyanine metallic complexes for catalytic reactions at CNRS in Lyon. I am currently working as CNRS research associate at the Chemistry Laboratory of ENS Lyon.
After a MSc degree at INSA Rouen, I moved to University of Strasbourg for a PhD supported by a MENRT fellowship. My doctoral work was focused on the light driven production of hydrogen and on the synthesis of novel fluorescent dyes. Then I worked on STM analyses of photoresponsive self-assembled monolayers as a JSPS fellow at Kyoto University, on redox-active molecular layers for electronic devices at Paris Diderot University and binuclear phthalocyanine metallic complexes for catalytic reactions at CNRS in Lyon. I am currently working as CNRS research associate at the Chemistry Laboratory of ENS Lyon.
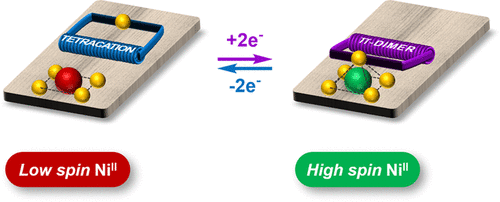
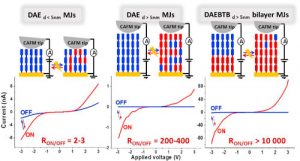
My research interests are directed towards π‐conjugated molecules with optical and electrochemical properties, their supramolecular self-assembly and functional materials with switchable properties (gels, liquid crystals, surfaces…).