I explore aspects of physical chemistry contribute to a sustainable development while preserving the environment. Physical chemical aspects related to solvation and molecular transport are key to optimize solvents and separation media and are essential to predict the environmental fate of chemical.
My current research interests concern the study of solvation, solubility and separations in alternative, environmentally friendly solvents, mainly ionic liquids, from a point of view of molecular thermodynamics.
Porous liquids, in particular porous ionic liquids, are promising novel materials for gas separations but also as reaction medium in catalytic reactions involving gaseous reactants.
GASES IN IONIC FLUIDS
I study the absorption of gases in ionic solvents – room temperature ionic liquids or their mixtures ( i.e. multi ionic liquids, mixtures with molecular compounds, eutectic mixtures).
The gases can be interesting to improve different separation processes, can be considered as models of complex solutes or can be used as molecular probes of the structure of the liquid solvent.
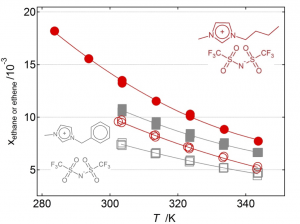
- Ethane/Ethylene Separation
Mole fraction solubility of ethane (empty symbols) and ethylene (full symbols) at 0.1 MPa partial pressure and as a function of temperature in two ionic liquids.
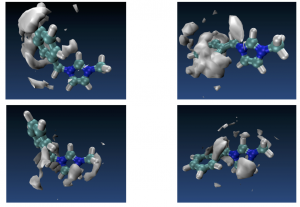
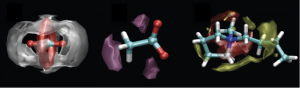
Spatial distribution functions of the center of mass of ethane (left hand-side) and ethylene (right hand-side) around the terminal atoms of the imidazolium cation. The surface corresponds to an iso-probability density 3.0 times the average of the system.
- Carbon dioxide capture by carboxylate-based ionic liquids
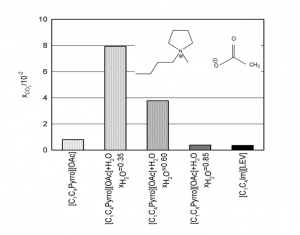
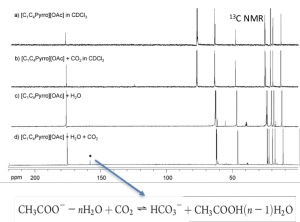
 Effect of water on the carbon dioxide absorption of imidazolium and pyrrolidinium acetate ionic liquids: a thermodynamic, spectroscopic and molecular simulation study
Effect of water on the carbon dioxide absorption of imidazolium and pyrrolidinium acetate ionic liquids: a thermodynamic, spectroscopic and molecular simulation study
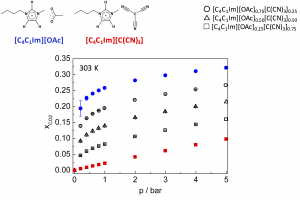 Carbon dioxide absorption in mixtures of two ionic liquids with a common cation
Carbon dioxide absorption in mixtures of two ionic liquids with a common cation
- Absorption of VOC in eutectic mixtures
POLYMERS IN IONIC LIQUIDS
- Poly(ethylene glycol) dimethyl ether in ammonium-based ionic liquids
POROUS IONIC LIQUIDS
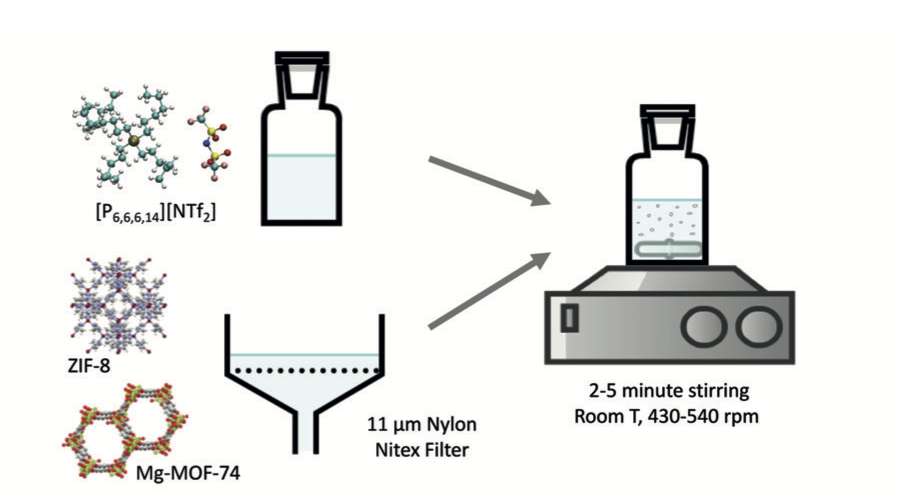
Porous ionic liquids can be prepared from the dispersion metal–organic frameworks (MOFs) in ionic liquids (ILs). Porous liquids prepared from 5 % of ZIF-8 in a phosphonium-based ionic liquid are capable of absorbing reversibly up to 150 % more nitrogen and 100 % more methane than the pure ionic liquid.
Preparation of phosphonium-based porous ionic liquids
When prepared from phosphonium carboxylate ionic liquids and ZIF-8, porous ionic liquids car absorb up to 100% more carbon dioxide than the pure MOF at low pressures.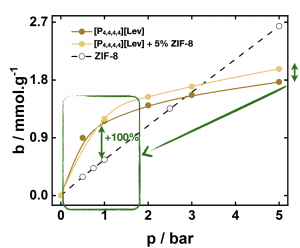 Tetrabutylphosphonium levulinate porous liquids absorb large amounts of CO2 at 1 bar
Tetrabutylphosphonium levulinate porous liquids absorb large amounts of CO2 at 1 bar