2022
Fluorescence-Amplified Detection of Redox Turnovers in Supported Lipid Bilayers Illuminates Redox Processes of α-Tocopherol
Aya Sakaya, Andrés M. Durantini, Yasser Gidi, Tara Šverko, Vincent Wieczny, Julia McCain & Gonzalo Cosa, ACS Appl. Mater. Interfaces 2022, 14, 11, 13872–13882
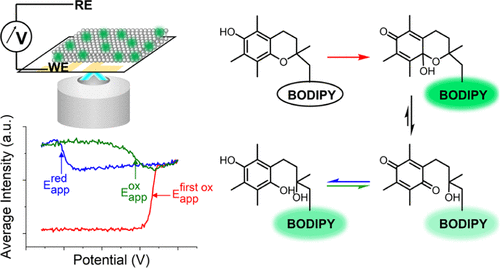
Abstract.
Electron-transfer processes in lipid membranes are key to biological functions, yet challenging to study because of the intrinsic heterogeneity of the systems. Here, we report spectro-electrochemical measurements on indium tin oxide-supported lipid bilayers toward the selective induction and sensing of redox processes in membranes. Working at neutral pH with a fluorogenic α-tocopherol analogue, the dynamics of the two-electron oxidation of the chromanol to a chromanone and the rapid thermal decay of the latter to a chromoquinone are recorded as a rapid surge and drop in intensity, respectively. Continuous voltage cycling reveals rapid chromoquinone two-electron, two-proton reduction to dihydrochromoquinone at negative bias, followed by slow regeneration of the former at positive bias. The kinetic parameters of these different transitions are readily obtained as a function of applied potentials. The sensitivity and selectivity afforded by the reported method enables monitoring signals equivalent to femtoampere currents with a high signal-to-background ratio. The study provides a new method to monitor membrane redox processes with high sensitivity and minimal concentrations and unravels key dynamic aspects of α-tocopherol redox chemistry.
Dynamic Electrochemiluminescence Imaging of Single Giant Liposome Opening at Polarized Electrode
Fatma Ben Trad, Vincent Wieczny, Jérôme Delacotte, Mathieu Morel, Manon Guille-Collignon, Stéphane Arbault, Frédéric Lemaître, Neso Sojic, Eric Labbé & Olivier Buriez, Anal. Chem. 2022, 94, 3, 1686–1696
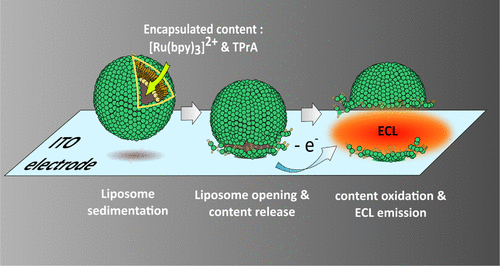
Abstract.
In this work, the characterization of release events from liposomes has been addressed quantitatively by an electrochemiluminescence (ECL) imaging strategy. First, ECL reagents ([Ru(bpy)3]2+ and tripropylamine) were encapsulated in sealed giant asymmetrical liposomes (100 μm in diameter) made of DOPG/DOPC phospholipids. After sedimentation on an indium tin oxide electrode material, the opening of liposomes was triggered by polarization of the surface. Under these conditions, amperometry, epifluorescence imaging, and ECL imaging were combined and synchronized to monitor and image the rupture of giant liposomes during the release and subsequent ECL emission of their redox content. Amperometry allowed the quantification of the content released from single liposomes. The location and status of liposomes (closed or opened) were assessed by epifluorescence imaging. ECL provided the image of the efflux of matter after liposome opening. This original ECL imaging approach favorably compares with strictly photoluminescent or electrochemical techniques and appears to be adapted for the investigation of membrane rupture/permeation events.