Redox-responsive 1D-assembly built from cucurbit[8]uril and a water-soluble metalloporphyrin-based tecton
Our article is available to read at J. Porphyr. Phthalocyanines.
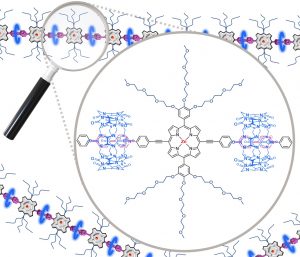 A linear porphyrin-based tecton bearing two 4,4′bipyridinium units (viologens) and two monomethyl-ether triethylene glycol-substituted phenyl substituents at the meso positions was synthesized and characterized. The latter was involved in the redox-triggered formation of linear supramolecular assemblies with cucurbit[8]uril (CB[8]) cavitands in aqueous media. The CB[8]-promoted intermolecular π-dimerization of the viologen cation radicals introduced at the meso positions of the porphyrin platform has been brought to light through the diagnostic signatures of the 1:2 host-guest ternary caviplexes formed between viologen and CB[8] and by spectroscopic data collected after electrochemical reduction of the viologen-based tectons.
A linear porphyrin-based tecton bearing two 4,4′bipyridinium units (viologens) and two monomethyl-ether triethylene glycol-substituted phenyl substituents at the meso positions was synthesized and characterized. The latter was involved in the redox-triggered formation of linear supramolecular assemblies with cucurbit[8]uril (CB[8]) cavitands in aqueous media. The CB[8]-promoted intermolecular π-dimerization of the viologen cation radicals introduced at the meso positions of the porphyrin platform has been brought to light through the diagnostic signatures of the 1:2 host-guest ternary caviplexes formed between viologen and CB[8] and by spectroscopic data collected after electrochemical reduction of the viologen-based tectons.