Dual-State Emissive π-Extended Salicylaldehyde Fluorophores: Synthesis, Photophysical Properties and First-Principle Calculations
Our article is available to read at Eur. J. Org. Chem.
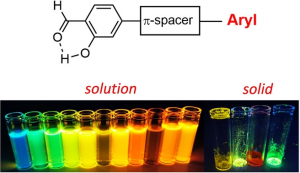 The search for simple, low-cost, versatile, easily accessible, stimuli-responsive, highly emissive molecular fluorophores emitting both in solution and in the solid-state has prompted us to investigate the optical properties of a series of synthetically accessible salicylaldehyde derivatives possessing a π-conjugated moiety at their 4-position. These dyes are mainly known as synthetic intermediates but can also display sizeable Excited-State Intramolecular Proton Transfer (ESIPT) fluorescence owing to the presence of a 6-membered H-bonded ring in their structure. The photophysical properties of these compounds have been studied in solution (multiple solvents) and in the solid-state, as doped in PMMA films, KBr pellets or as powders leading to the observation of a pronounced fluorosolvatochromism. Emission wavelengths in the range 400–654 nm, along with photoluminescence quantum yields up to 76 % were recorded. Modification of the spacer (ethynyl, vinyl or direct connection) involved the π-delocalization triggers major differences in terms of maximum emission wavelength and fluorescence quantum yields in the various media studied. All photophysical observations are rationalized by first-principle calculations.
The search for simple, low-cost, versatile, easily accessible, stimuli-responsive, highly emissive molecular fluorophores emitting both in solution and in the solid-state has prompted us to investigate the optical properties of a series of synthetically accessible salicylaldehyde derivatives possessing a π-conjugated moiety at their 4-position. These dyes are mainly known as synthetic intermediates but can also display sizeable Excited-State Intramolecular Proton Transfer (ESIPT) fluorescence owing to the presence of a 6-membered H-bonded ring in their structure. The photophysical properties of these compounds have been studied in solution (multiple solvents) and in the solid-state, as doped in PMMA films, KBr pellets or as powders leading to the observation of a pronounced fluorosolvatochromism. Emission wavelengths in the range 400–654 nm, along with photoluminescence quantum yields up to 76 % were recorded. Modification of the spacer (ethynyl, vinyl or direct connection) involved the π-delocalization triggers major differences in terms of maximum emission wavelength and fluorescence quantum yields in the various media studied. All photophysical observations are rationalized by first-principle calculations.