Our article is available to read at Chem. Sci.

Our article is available to read at Chem. Sci.

Our article is available to read at Soft Matter.
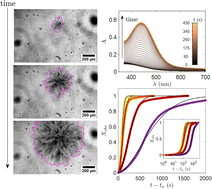 Supramolecular crystal gels, a subset of molecular gels, are formed through the self-assembly of low molecular weight gelators into interconnecting crystalline fibers, creating a three-dimensional soft solid network. This study focuses on the formation and properties of viologen-based supramolecular crystalline gels. It aims to answer key questions about the tunability of network properties and the origin of these properties through in-depth analyses of the gelation kinetics triggered by thermal quenching. Experimental investigations, including UV-Vis absorption spectroscopy, rheology, microscopy and scattering measurements, contribute to a comprehensive and self-consistent understanding of the system kinetics. We confirm that viologen-based gelators crystallize by forming nanometer radius hollow tubes that assemble into micro to millimetric spherulites. We then show that crystallization follows the Avrami theory and is based on pre-existing nuclei. We also establish that the growth is interface-controlled, leading the hollow tubes to branch into spherulites with fractal structures. Finally, we demonstrate that the gel properties can be tuned depending on the quenching temperature. Lowering the temperature results in the formation of denser and smaller spherulites. In contrast, the gel’s elasticity is not significantly affected by the quench temperature, leading us to hypothesize that the densification of spherulites occurs at the expense of connectivity between spherulites.
Supramolecular crystal gels, a subset of molecular gels, are formed through the self-assembly of low molecular weight gelators into interconnecting crystalline fibers, creating a three-dimensional soft solid network. This study focuses on the formation and properties of viologen-based supramolecular crystalline gels. It aims to answer key questions about the tunability of network properties and the origin of these properties through in-depth analyses of the gelation kinetics triggered by thermal quenching. Experimental investigations, including UV-Vis absorption spectroscopy, rheology, microscopy and scattering measurements, contribute to a comprehensive and self-consistent understanding of the system kinetics. We confirm that viologen-based gelators crystallize by forming nanometer radius hollow tubes that assemble into micro to millimetric spherulites. We then show that crystallization follows the Avrami theory and is based on pre-existing nuclei. We also establish that the growth is interface-controlled, leading the hollow tubes to branch into spherulites with fractal structures. Finally, we demonstrate that the gel properties can be tuned depending on the quenching temperature. Lowering the temperature results in the formation of denser and smaller spherulites. In contrast, the gel’s elasticity is not significantly affected by the quench temperature, leading us to hypothesize that the densification of spherulites occurs at the expense of connectivity between spherulites.
Our article is available to read at J. Phys. Chem. C.
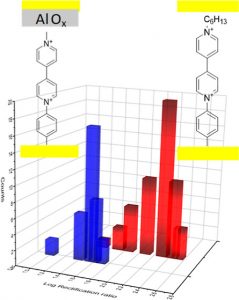 In the present work, two viologen derivatives were synthesized and used in molecular junctions (MJs) to investigate the impact of a decoupling unit on the rectification behavior. Both bear an aniline terminal unit but one of the viologen derivative (named VIO-C1) is terminated by a methyl group whereas the other (named VIO-C6) bears a six-carbon (C6H13) terminal chain. Thin layers of each derivative were deposited on gold microelectrodes by the electroreduction of the corresponding diazonium reagents, and solid-state MJs were fabricated to study the electronic transport. JV curves of VIO-C1 show a symmetric behavior, whereas the VIO-C6 (with alkyl chain) JV curves show strong asymmetry in the transport. This behavior is attributed to the decrease of the electronic coupling at the top interface due to the alkyl chain and is confirmed by comparison with similar VIO-C1 MJs in which a thin AlOx layer is inserted on top of the VIO-C1 layer. The impact of this decoupling unit on the rectification ratio is discussed.
In the present work, two viologen derivatives were synthesized and used in molecular junctions (MJs) to investigate the impact of a decoupling unit on the rectification behavior. Both bear an aniline terminal unit but one of the viologen derivative (named VIO-C1) is terminated by a methyl group whereas the other (named VIO-C6) bears a six-carbon (C6H13) terminal chain. Thin layers of each derivative were deposited on gold microelectrodes by the electroreduction of the corresponding diazonium reagents, and solid-state MJs were fabricated to study the electronic transport. JV curves of VIO-C1 show a symmetric behavior, whereas the VIO-C6 (with alkyl chain) JV curves show strong asymmetry in the transport. This behavior is attributed to the decrease of the electronic coupling at the top interface due to the alkyl chain and is confirmed by comparison with similar VIO-C1 MJs in which a thin AlOx layer is inserted on top of the VIO-C1 layer. The impact of this decoupling unit on the rectification ratio is discussed.
Our article is available to read at J. Mater. Chem. C.
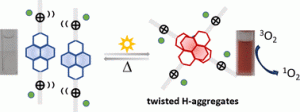 Naphthalenediimide amphiphiles (NDI-as) with quaternary ammonium groups (DC4, DaP, and DaO) display unprecedented UV light-induced aggregation in solutions of water, acetonitrile and THF. Light-induced aggregation brings significant spectral modification by which unusual absorption band distribution is assigned to H-type aggregates. Such aggregation can be reversibly broken by heating and reassembled by photoirradiation. The irradiation/heating cycles that provoke assembly/disassembly process can be confirmed by 1H-NMR spectroscopy. The possibility of a chemically promoted aggregation by or radical anion either anion–π interaction may be ruled out by UV-Vis, EPR and electrochemistry measurements. These photoinduced aggregates can sensitize dissolved O2 to produce singlet oxygen, a surprising observation when it comes to sensitization efficiency for dyes as aggregation usually decreases sensitization yield. Laser-induced luminescence measurements corroborate the existence of monomeric and aggregate forms, as well as confirm triplet states as phosphorescence was detected, being particularly intense in concentrated THF solutions.
Naphthalenediimide amphiphiles (NDI-as) with quaternary ammonium groups (DC4, DaP, and DaO) display unprecedented UV light-induced aggregation in solutions of water, acetonitrile and THF. Light-induced aggregation brings significant spectral modification by which unusual absorption band distribution is assigned to H-type aggregates. Such aggregation can be reversibly broken by heating and reassembled by photoirradiation. The irradiation/heating cycles that provoke assembly/disassembly process can be confirmed by 1H-NMR spectroscopy. The possibility of a chemically promoted aggregation by or radical anion either anion–π interaction may be ruled out by UV-Vis, EPR and electrochemistry measurements. These photoinduced aggregates can sensitize dissolved O2 to produce singlet oxygen, a surprising observation when it comes to sensitization efficiency for dyes as aggregation usually decreases sensitization yield. Laser-induced luminescence measurements corroborate the existence of monomeric and aggregate forms, as well as confirm triplet states as phosphorescence was detected, being particularly intense in concentrated THF solutions.
Our article is available to read at J. Mater. Chem. C.
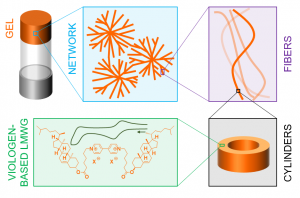 Redox-active conductive supramolecular gels involving highly ordered chiral assemblies of small organic molecules are very promising soft materials for many applications ranging from catalysis to electronics. However, combining all these properties in the same material has so far remained a difficult task. We now report the synthesis and detailed structural, rheological, and electrical characterizations of supramolecular gels obtained by self-assembly of a dicationic low molecular weight gelator incorporating a redox-active 4,4′-bipyridinium unit. These molecules have been shown to self-assemble in pentanol to form chiral hollow core–shell cylinders, eventually yielding dendritic clusters inducing gelation. We also showed that the optical, rheological, and electrical properties of the gels can be tuned by adding ionic additives. Careful control of the formation of charge-transfer complexes between viologens and iodides has led to the formation of robust, transparent, conductive, and chiral gel. The gelation process, the properties of the gel, and the structure of the assemblies have been thoroughly investigated by UV-Vis and ECD spectroscopy, rheometry, bright-field microscopy, SAXS, AFM, electrochemical and impedance measurements.
Redox-active conductive supramolecular gels involving highly ordered chiral assemblies of small organic molecules are very promising soft materials for many applications ranging from catalysis to electronics. However, combining all these properties in the same material has so far remained a difficult task. We now report the synthesis and detailed structural, rheological, and electrical characterizations of supramolecular gels obtained by self-assembly of a dicationic low molecular weight gelator incorporating a redox-active 4,4′-bipyridinium unit. These molecules have been shown to self-assemble in pentanol to form chiral hollow core–shell cylinders, eventually yielding dendritic clusters inducing gelation. We also showed that the optical, rheological, and electrical properties of the gels can be tuned by adding ionic additives. Careful control of the formation of charge-transfer complexes between viologens and iodides has led to the formation of robust, transparent, conductive, and chiral gel. The gelation process, the properties of the gel, and the structure of the assemblies have been thoroughly investigated by UV-Vis and ECD spectroscopy, rheometry, bright-field microscopy, SAXS, AFM, electrochemical and impedance measurements.
Our article is available to read at J. Porphyr. Phthalocyanines.
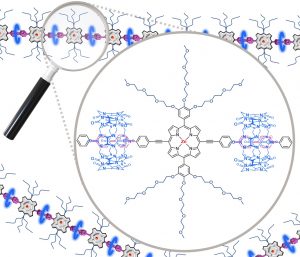 A linear porphyrin-based tecton bearing two 4,4′bipyridinium units (viologens) and two monomethyl-ether triethylene glycol-substituted phenyl substituents at the meso positions was synthesized and characterized. The latter was involved in the redox-triggered formation of linear supramolecular assemblies with cucurbit[8]uril (CB[8]) cavitands in aqueous media. The CB[8]-promoted intermolecular π-dimerization of the viologen cation radicals introduced at the meso positions of the porphyrin platform has been brought to light through the diagnostic signatures of the 1:2 host-guest ternary caviplexes formed between viologen and CB[8] and by spectroscopic data collected after electrochemical reduction of the viologen-based tectons.
A linear porphyrin-based tecton bearing two 4,4′bipyridinium units (viologens) and two monomethyl-ether triethylene glycol-substituted phenyl substituents at the meso positions was synthesized and characterized. The latter was involved in the redox-triggered formation of linear supramolecular assemblies with cucurbit[8]uril (CB[8]) cavitands in aqueous media. The CB[8]-promoted intermolecular π-dimerization of the viologen cation radicals introduced at the meso positions of the porphyrin platform has been brought to light through the diagnostic signatures of the 1:2 host-guest ternary caviplexes formed between viologen and CB[8] and by spectroscopic data collected after electrochemical reduction of the viologen-based tectons.
 A PhD position is opened at the Chemistry Laboratory of ENS de Lyon and funded for three years by the French ANR in the frame of the project ChiroSwitch: metamorphic approaches for on-surface switching of chiroptical properties.
A PhD position is opened at the Chemistry Laboratory of ENS de Lyon and funded for three years by the French ANR in the frame of the project ChiroSwitch: metamorphic approaches for on-surface switching of chiroptical properties.
More informations here.
Our article is available to read at J. Am. Chem. Soc.
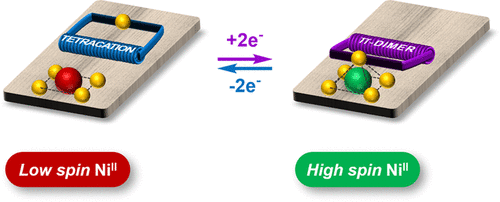 We herein report the synthesis and magnetic properties of a Ni(II)-porphyrin tethered to an imidazole ligand through a flexible electron-responsive mechanical hinge. The latter is capable of undergoing a large amplitude and fully reversible folding motion under the effect of electrical stimulation. This redox-triggered movement is exploited to force the axial coordination of the appended imidazole ligand onto the square-planar Ni(II) center, resulting in a change in its spin state from low spin (S = 0) to high spin (S = 1) proceeding with an 80% switching efficiency. The driving force of this reversible folding motion is the π-dimerization between two electrogenerated viologen cation radicals. The folding motion and the associated spin state switching are demonstrated on the grounds of NMR, (spectro)electrochemical, and magnetic data supported by quantum calculations.
We herein report the synthesis and magnetic properties of a Ni(II)-porphyrin tethered to an imidazole ligand through a flexible electron-responsive mechanical hinge. The latter is capable of undergoing a large amplitude and fully reversible folding motion under the effect of electrical stimulation. This redox-triggered movement is exploited to force the axial coordination of the appended imidazole ligand onto the square-planar Ni(II) center, resulting in a change in its spin state from low spin (S = 0) to high spin (S = 1) proceeding with an 80% switching efficiency. The driving force of this reversible folding motion is the π-dimerization between two electrogenerated viologen cation radicals. The folding motion and the associated spin state switching are demonstrated on the grounds of NMR, (spectro)electrochemical, and magnetic data supported by quantum calculations.
Our article is available to read at ECS J. Solid State Sci. Technol.
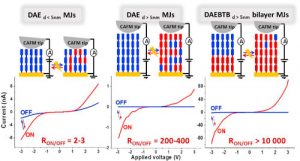 Through electrochemical deposition, photoswitchable single and bilayer molecular junctions based on diarylethene (DAE) and bisthienylbenzene (BTB) layers were fabricated. The electrical characterization of closed and open forms of DAE were investigated by C-AFM for two different layer thicknesses fixed at 2–3 nm and 8–9 nm, i.e. below and above the direct tunneling limit. Both layers switch between high and low conductance modes (« ON » and « OFF » states) when irradiated by UV and visible light. ON/OFF ratios of 2–3 and 200–400 were obtained for 3 nm- and 9 nm-thick DAE MJs, respectively. Next, we prepared 9 nm-thick MJs using a bi-layer system. The first layer (5 nm) is based on BTB oligomers. The second layer (4 nm) is based on DAE oligomers. The impact of this first layer on the switchable properties of the system, and on the photoresponse of the 9 nm-thick DAE-based MJs has been studied. The DAE/BTB bilayer generates new electronic functions combining photoswitching and photorectification. The open form of DAE/BTB shows low conductance and asymmetric I(V) curves while the closed form shows symmetric I(V) curves and high conductance. More importantly, unprecedented ON/OFF current ratios of over 10 000 at 1 volt were reproducibly measured.
Through electrochemical deposition, photoswitchable single and bilayer molecular junctions based on diarylethene (DAE) and bisthienylbenzene (BTB) layers were fabricated. The electrical characterization of closed and open forms of DAE were investigated by C-AFM for two different layer thicknesses fixed at 2–3 nm and 8–9 nm, i.e. below and above the direct tunneling limit. Both layers switch between high and low conductance modes (« ON » and « OFF » states) when irradiated by UV and visible light. ON/OFF ratios of 2–3 and 200–400 were obtained for 3 nm- and 9 nm-thick DAE MJs, respectively. Next, we prepared 9 nm-thick MJs using a bi-layer system. The first layer (5 nm) is based on BTB oligomers. The second layer (4 nm) is based on DAE oligomers. The impact of this first layer on the switchable properties of the system, and on the photoresponse of the 9 nm-thick DAE-based MJs has been studied. The DAE/BTB bilayer generates new electronic functions combining photoswitching and photorectification. The open form of DAE/BTB shows low conductance and asymmetric I(V) curves while the closed form shows symmetric I(V) curves and high conductance. More importantly, unprecedented ON/OFF current ratios of over 10 000 at 1 volt were reproducibly measured.
Our article is available to read at ECS Adv.
 Viologen-based ditopic bis-pyridinyl-triazole bidentate ligands self-assemble in the presence of palladium ions into supramolecular polymers whose structure is imposed by the directed formation of coordination bonds. Light-irradiation of these electron-responsive supramolecular materials triggers a photo-induced electron transfer yielding isolated π-radicals and dimers of radicals. The photoreduction events and the associated dimerization steps trigger a large-scale reorganization occurring within the supramolecular network yielding aggregates or gels depending on the irradiation conditions (power, duration). Detailed electrochemical, spectro-electrochemical and photochemical analyses were conducted to understand the mechanisms at stakes in these light-induced aggregation and gelation.
Viologen-based ditopic bis-pyridinyl-triazole bidentate ligands self-assemble in the presence of palladium ions into supramolecular polymers whose structure is imposed by the directed formation of coordination bonds. Light-irradiation of these electron-responsive supramolecular materials triggers a photo-induced electron transfer yielding isolated π-radicals and dimers of radicals. The photoreduction events and the associated dimerization steps trigger a large-scale reorganization occurring within the supramolecular network yielding aggregates or gels depending on the irradiation conditions (power, duration). Detailed electrochemical, spectro-electrochemical and photochemical analyses were conducted to understand the mechanisms at stakes in these light-induced aggregation and gelation.