Our article is available to read at J. Org. Chem.
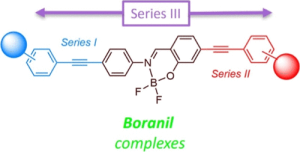 We describe herein the synthesis along with full photophysical and computational studies of three series of boranil complexes (Series I−III), incorporating extended ethynyl substitution, either on the imino side (Series I), on the phenolic side (Series II), or on both sides (Series III), in order to assess the impact of the insertion of electron donors or acceptors on the fluorescence properties. A full photophysical study in solution (various solvents) demonstrated the presence of a strong intramolecular charge transfer process within these boron complexes, with fluorescence colors spanning the entire visible range. Additionally, these compounds are also emissive in the solid state, on a similar wide fluorescence range, thus providing more examples of dual solution- and solid-emissive fluorophores, based on boron complexes. Theoretical calculations on both anil ligands and boranil complexes rationalize the charge transfer nature of the excited states involved in the emissive transitions.
We describe herein the synthesis along with full photophysical and computational studies of three series of boranil complexes (Series I−III), incorporating extended ethynyl substitution, either on the imino side (Series I), on the phenolic side (Series II), or on both sides (Series III), in order to assess the impact of the insertion of electron donors or acceptors on the fluorescence properties. A full photophysical study in solution (various solvents) demonstrated the presence of a strong intramolecular charge transfer process within these boron complexes, with fluorescence colors spanning the entire visible range. Additionally, these compounds are also emissive in the solid state, on a similar wide fluorescence range, thus providing more examples of dual solution- and solid-emissive fluorophores, based on boron complexes. Theoretical calculations on both anil ligands and boranil complexes rationalize the charge transfer nature of the excited states involved in the emissive transitions.
 Herein we report one of the very first supramolecular gel for which the formation and dissociation of the self-assembled network can be achieved using an electrochemical stimulation. The redox-triggered macroscopic changes under different photochemical or electrochemical stimuli result from the reversible assembly of charge transfer complexes formed between the viologen acceptor and iodide counter-anions.
Herein we report one of the very first supramolecular gel for which the formation and dissociation of the self-assembled network can be achieved using an electrochemical stimulation. The redox-triggered macroscopic changes under different photochemical or electrochemical stimuli result from the reversible assembly of charge transfer complexes formed between the viologen acceptor and iodide counter-anions.
 After a MSc degree at INSA Rouen, I moved to University of Strasbourg for a PhD supported by a MENRT fellowship. My doctoral work was focused on the light driven production of hydrogen and on the synthesis of novel fluorescent dyes. Then I worked on STM analyses of photoresponsive self-assembled monolayers as a JSPS fellow at Kyoto University, on redox-active molecular layers for electronic devices at Paris Diderot University and binuclear phthalocyanine metallic complexes for catalytic reactions at CNRS in Lyon. I am currently working as CNRS research associate at the Chemistry Laboratory of ENS Lyon.
After a MSc degree at INSA Rouen, I moved to University of Strasbourg for a PhD supported by a MENRT fellowship. My doctoral work was focused on the light driven production of hydrogen and on the synthesis of novel fluorescent dyes. Then I worked on STM analyses of photoresponsive self-assembled monolayers as a JSPS fellow at Kyoto University, on redox-active molecular layers for electronic devices at Paris Diderot University and binuclear phthalocyanine metallic complexes for catalytic reactions at CNRS in Lyon. I am currently working as CNRS research associate at the Chemistry Laboratory of ENS Lyon.