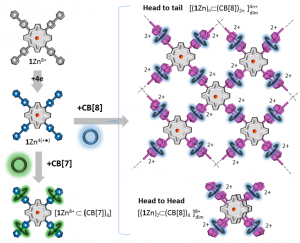
Our article is available to read at Nanoscale
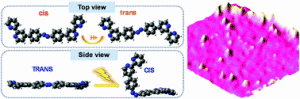 Ligands are designed to have ditopic bipyridine terminal groups linked through photochromic azobenzene central units, which exhibit multi-switchable properties by different external stimuli. The molecule can switch between cis-and trans-conformations at their bipyridine terminal groups upon protonation and at their central azobenzene units upon irradiation of photons. As a result, the system shows four different isomeric states: cis–TRANS, trans–TRANS, cis–CIS and trans–CIS. The four conformers are switched and are visualized by scanning tunneling microscopy at the solid–liquid interface, which gives a direct demonstration of the multi-functional switches at a molecular level.
Ligands are designed to have ditopic bipyridine terminal groups linked through photochromic azobenzene central units, which exhibit multi-switchable properties by different external stimuli. The molecule can switch between cis-and trans-conformations at their bipyridine terminal groups upon protonation and at their central azobenzene units upon irradiation of photons. As a result, the system shows four different isomeric states: cis–TRANS, trans–TRANS, cis–CIS and trans–CIS. The four conformers are switched and are visualized by scanning tunneling microscopy at the solid–liquid interface, which gives a direct demonstration of the multi-functional switches at a molecular level.