Research
 My research interests at the Chemistry Laboratory of ENS Lyon are directed towards π-conjugated molecules with optical and electrochemical properties, their supramolecular self-assembly and functional materials with switchable properties (gels, liquid crystals, surfaces…).
My research interests at the Chemistry Laboratory of ENS Lyon are directed towards π-conjugated molecules with optical and electrochemical properties, their supramolecular self-assembly and functional materials with switchable properties (gels, liquid crystals, surfaces…).
Selected articles
Reversible Self-assembly of a Viologen-based Supramolecular Gel Network under Electrochemical Control
Our article is available to read at Chem. Eur. J.
 Herein we report one of the very first supramolecular gel for which the formation and dissociation of the self-assembled network can be achieved using an electrochemical stimulation. The redox-triggered macroscopic changes under different photochemical or electrochemical stimuli result from the reversible assembly of charge transfer complexes formed between the viologen acceptor and iodide counter-anions.
Herein we report one of the very first supramolecular gel for which the formation and dissociation of the self-assembled network can be achieved using an electrochemical stimulation. The redox-triggered macroscopic changes under different photochemical or electrochemical stimuli result from the reversible assembly of charge transfer complexes formed between the viologen acceptor and iodide counter-anions.
π-Expansion as gateway to viologen-based pimers
Our article is available to read at Chem. Sci.

Chiral and conductive viologen-based supramolecular gels exhibiting tunable charge-transfer properties
Our article is available to read at J. Mater. Chem. C.
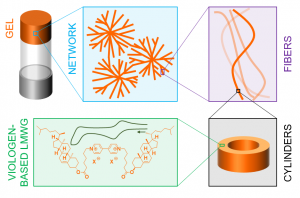 Redox-active conductive supramolecular gels involving highly ordered chiral assemblies of small organic molecules are very promising soft materials for many applications ranging from catalysis to electronics. However, combining all these properties in the same material has so far remained a difficult task. We now report the synthesis and detailed structural, rheological, and electrical characterizations of supramolecular gels obtained by self-assembly of a dicationic low molecular weight gelator incorporating a redox-active 4,4′-bipyridinium unit. These molecules have been shown to self-assemble in pentanol to form chiral hollow core–shell cylinders, eventually yielding dendritic clusters inducing gelation. We also showed that the optical, rheological, and electrical properties of the gels can be tuned by adding ionic additives. Careful control of the formation of charge-transfer complexes between viologens and iodides has led to the formation of robust, transparent, conductive, and chiral gel. The gelation process, the properties of the gel, and the structure of the assemblies have been thoroughly investigated by UV-Vis and ECD spectroscopy, rheometry, bright-field microscopy, SAXS, AFM, electrochemical and impedance measurements.
Redox-active conductive supramolecular gels involving highly ordered chiral assemblies of small organic molecules are very promising soft materials for many applications ranging from catalysis to electronics. However, combining all these properties in the same material has so far remained a difficult task. We now report the synthesis and detailed structural, rheological, and electrical characterizations of supramolecular gels obtained by self-assembly of a dicationic low molecular weight gelator incorporating a redox-active 4,4′-bipyridinium unit. These molecules have been shown to self-assemble in pentanol to form chiral hollow core–shell cylinders, eventually yielding dendritic clusters inducing gelation. We also showed that the optical, rheological, and electrical properties of the gels can be tuned by adding ionic additives. Careful control of the formation of charge-transfer complexes between viologens and iodides has led to the formation of robust, transparent, conductive, and chiral gel. The gelation process, the properties of the gel, and the structure of the assemblies have been thoroughly investigated by UV-Vis and ECD spectroscopy, rheometry, bright-field microscopy, SAXS, AFM, electrochemical and impedance measurements.
Photoredox Processes in the Aggregation and Gelation of Electron-Responsive Supramolecular Polymers Based on Viologen
Our article is available to read at ECS Adv.
 Viologen-based ditopic bis-pyridinyl-triazole bidentate ligands self-assemble in the presence of palladium ions into supramolecular polymers whose structure is imposed by the directed formation of coordination bonds. Light-irradiation of these electron-responsive supramolecular materials triggers a photo-induced electron transfer yielding isolated π-radicals and dimers of radicals. The photoreduction events and the associated dimerization steps trigger a large-scale reorganization occurring within the supramolecular network yielding aggregates or gels depending on the irradiation conditions (power, duration). Detailed electrochemical, spectro-electrochemical and photochemical analyses were conducted to understand the mechanisms at stakes in these light-induced aggregation and gelation.
Viologen-based ditopic bis-pyridinyl-triazole bidentate ligands self-assemble in the presence of palladium ions into supramolecular polymers whose structure is imposed by the directed formation of coordination bonds. Light-irradiation of these electron-responsive supramolecular materials triggers a photo-induced electron transfer yielding isolated π-radicals and dimers of radicals. The photoreduction events and the associated dimerization steps trigger a large-scale reorganization occurring within the supramolecular network yielding aggregates or gels depending on the irradiation conditions (power, duration). Detailed electrochemical, spectro-electrochemical and photochemical analyses were conducted to understand the mechanisms at stakes in these light-induced aggregation and gelation.
Photo/Redox-Responsive 2D-Supramolecular Assembly Involving Cucurbit[8]uril and a Star-Shaped Porphyrin Tecton
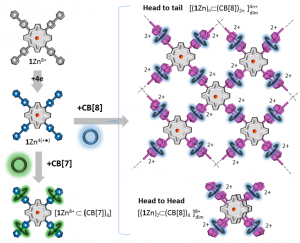 The present paper reports on the formation and on the electrochemical/spectroscopic characterization of inclusion complexes formed in aqueous media between cucurbit[7 or 8]urils cavitands (CB[7], CB[8]) and a rigid four-pointed star-shaped viologen-appended porphyrin tecton. The formation of discrete 4:1 pseudo-rotaxane-like caviplexes, involving threading of CB[n] rings on the rigid viologen-based star’s branches has been demonstrated by nuclear magnetic resonance and mass spectrometry measurements. Then, the photo- and redox-triggered formation of 2D supramolecular assemblies involving CB[8]s and the four electron reduced tectons as key building elements, has been established on the ground of in-depth electrochemical and spectroscopic analyses supported by quantum calculations. The CB[8]-promoted intermolecular π-dimerization of the viologen cation radicals introduced at the meso positions of the porphyrin plateform has been brought to light through the diagnostic signatures of the 1:2 host-guest ternary caviplexes formed between viologen and CB[8] and by spectroscopic data collected after electrochemical, chemical or photochemical reduction of the viologen-based tectons. The CB[8] hosts not only proved useful to promote the redox-triggered formation of supramolecular assemblies, it was also found to prevent the chemical reduction of the porphyrin ring in aqueous media and its subsequent conversion into phlorin products.
The present paper reports on the formation and on the electrochemical/spectroscopic characterization of inclusion complexes formed in aqueous media between cucurbit[7 or 8]urils cavitands (CB[7], CB[8]) and a rigid four-pointed star-shaped viologen-appended porphyrin tecton. The formation of discrete 4:1 pseudo-rotaxane-like caviplexes, involving threading of CB[n] rings on the rigid viologen-based star’s branches has been demonstrated by nuclear magnetic resonance and mass spectrometry measurements. Then, the photo- and redox-triggered formation of 2D supramolecular assemblies involving CB[8]s and the four electron reduced tectons as key building elements, has been established on the ground of in-depth electrochemical and spectroscopic analyses supported by quantum calculations. The CB[8]-promoted intermolecular π-dimerization of the viologen cation radicals introduced at the meso positions of the porphyrin plateform has been brought to light through the diagnostic signatures of the 1:2 host-guest ternary caviplexes formed between viologen and CB[8] and by spectroscopic data collected after electrochemical, chemical or photochemical reduction of the viologen-based tectons. The CB[8] hosts not only proved useful to promote the redox-triggered formation of supramolecular assemblies, it was also found to prevent the chemical reduction of the porphyrin ring in aqueous media and its subsequent conversion into phlorin products.
Electrografted Monolayer Based on a Naphtalenediimide-Ruthenium Terpyridine Complex Dyad: Efficient Creation of Large-area Molecular Junctions with High Current Densities
Our article is available to read at Chem. Commun.
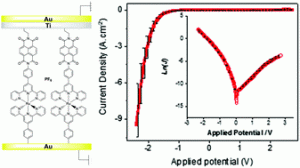 An electron donor–acceptor dyad has been designed for the creation of large-area molecular junctions (MJ). Diazonium cation electrografting was used to form well controlled monolayers. The robustness of the monolayer enabled the creation of MJs using direct top-coat evaporation with a high yield of operating devices.
An electron donor–acceptor dyad has been designed for the creation of large-area molecular junctions (MJ). Diazonium cation electrografting was used to form well controlled monolayers. The robustness of the monolayer enabled the creation of MJs using direct top-coat evaporation with a high yield of operating devices.
Vectorization and Intracellular Distribution of a Two Photon- Absorbing, Near Infra-Red (NIR) Emitting π-Extended Boranil Dye
Our article is available to read at ChemPhotoChem
 A π‐extended boranil (PEB) dye has been synthesized and its photophysical properties have been recorded in a range of solvents. The dye exhibits an intense solvatochromic fluorescence emission covering the entire visible spectrum and the near infra‐red (NIR) region (from λ=534 to 764 nm) with quantum yields up to 69 %. Embedment of the PEB dye in the amphiphilic block copolymer Cremophor EL® leads to a strong emission (λem=617 nm, Φ=0.33) in PBS buffer. Moreover, two‐photon absorption (TPA) spectroscopy evidenced a large two‐photon (2P) cross section (420 GM), enabling the use of the PEB dye in 2P‐excitation fluorescence microscopy. The cellular uptake and distribution of PEB in HeLa cells was monitored using fluorescence lifetime imaging microscopy (FLIM) and real‐time widefield imaging. It was found that the emission of the dye is strongly influenced by the local environment and that the dye is preferentially internalized in acidic vesicles.
A π‐extended boranil (PEB) dye has been synthesized and its photophysical properties have been recorded in a range of solvents. The dye exhibits an intense solvatochromic fluorescence emission covering the entire visible spectrum and the near infra‐red (NIR) region (from λ=534 to 764 nm) with quantum yields up to 69 %. Embedment of the PEB dye in the amphiphilic block copolymer Cremophor EL® leads to a strong emission (λem=617 nm, Φ=0.33) in PBS buffer. Moreover, two‐photon absorption (TPA) spectroscopy evidenced a large two‐photon (2P) cross section (420 GM), enabling the use of the PEB dye in 2P‐excitation fluorescence microscopy. The cellular uptake and distribution of PEB in HeLa cells was monitored using fluorescence lifetime imaging microscopy (FLIM) and real‐time widefield imaging. It was found that the emission of the dye is strongly influenced by the local environment and that the dye is preferentially internalized in acidic vesicles.
Diarylethene Self-Assembled Monolayers: Cocrystallization and Mixing-Induced Cooperativity Highlighted by Scanning Tunneling Microscopy at the Liquid/Solid Interface
Our article is available to read at Chem. Eur. J.
 Stimulus control over 2D multicomponent molecular ordering on surfaces is a key technique for realizing advanced materials with stimuli‐responsive surface properties. The formation of 2D molecular ordering along with photoisomerization was monitored by scanning tunneling microscopy at the octanoic acid/highly oriented pyrolytic graphite interface for a synthesized amide‐containing diarylethene, which underwent photoisomerization between the open‐ and closed‐ring isomers and also a side‐reaction to give the annulated isomer. The nucleation (Kn) and elongation (Ke) equilibrium constants were determined by analysis of the concentration dependence of the surface coverage by using a cooperative model at the liquid/solid interface. It was found that the annulated isomer has a very large equilibrium constant, which explains the predominantly observed ordering of the annulated isomer. It was also found that the presence of the closed‐ring isomer induces cooperativity into the formation of molecular ordering composed of the open‐ring isomer. A quantitative analysis of the formation of ordering by using the cooperative model has provided a new view of the formation of 2D multicomponent molecular ordering.
Stimulus control over 2D multicomponent molecular ordering on surfaces is a key technique for realizing advanced materials with stimuli‐responsive surface properties. The formation of 2D molecular ordering along with photoisomerization was monitored by scanning tunneling microscopy at the octanoic acid/highly oriented pyrolytic graphite interface for a synthesized amide‐containing diarylethene, which underwent photoisomerization between the open‐ and closed‐ring isomers and also a side‐reaction to give the annulated isomer. The nucleation (Kn) and elongation (Ke) equilibrium constants were determined by analysis of the concentration dependence of the surface coverage by using a cooperative model at the liquid/solid interface. It was found that the annulated isomer has a very large equilibrium constant, which explains the predominantly observed ordering of the annulated isomer. It was also found that the presence of the closed‐ring isomer induces cooperativity into the formation of molecular ordering composed of the open‐ring isomer. A quantitative analysis of the formation of ordering by using the cooperative model has provided a new view of the formation of 2D multicomponent molecular ordering.
Luminescent materials: Locking π-conjugated and heterocyclic ligands with boron (III).
Our article is available to read at Angew. Chem. Int. Ed.
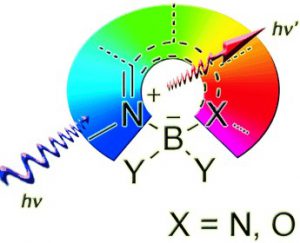 Multidisciplinary research on novel organic luminescent dyes is propelled by potential applications in plastic electronics and biomedical sciences. The construction of sophisticated fluorescent dyes around a tetrahedral boron(III) center is a particular approach that has fueled the creativity of chemists. Success in this enterprise has been readily achieved with simple synthetic protocols, the products of which display unusual spectroscopic behavior. This account is a critical review of recent advances in the field of boron(III) complexes (excluding BODIPYs and acetylacetonate boron complexes) involving species displaying similar coordination features, and we outline their potential development in several disciplines.
Multidisciplinary research on novel organic luminescent dyes is propelled by potential applications in plastic electronics and biomedical sciences. The construction of sophisticated fluorescent dyes around a tetrahedral boron(III) center is a particular approach that has fueled the creativity of chemists. Success in this enterprise has been readily achieved with simple synthetic protocols, the products of which display unusual spectroscopic behavior. This account is a critical review of recent advances in the field of boron(III) complexes (excluding BODIPYs and acetylacetonate boron complexes) involving species displaying similar coordination features, and we outline their potential development in several disciplines.
