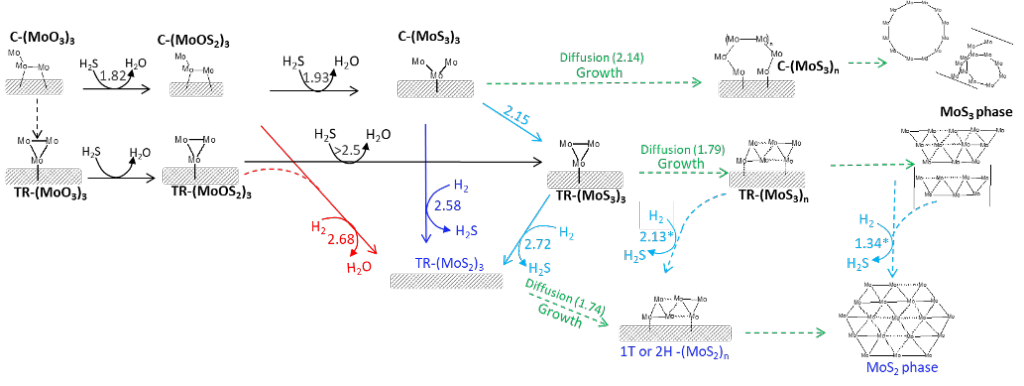
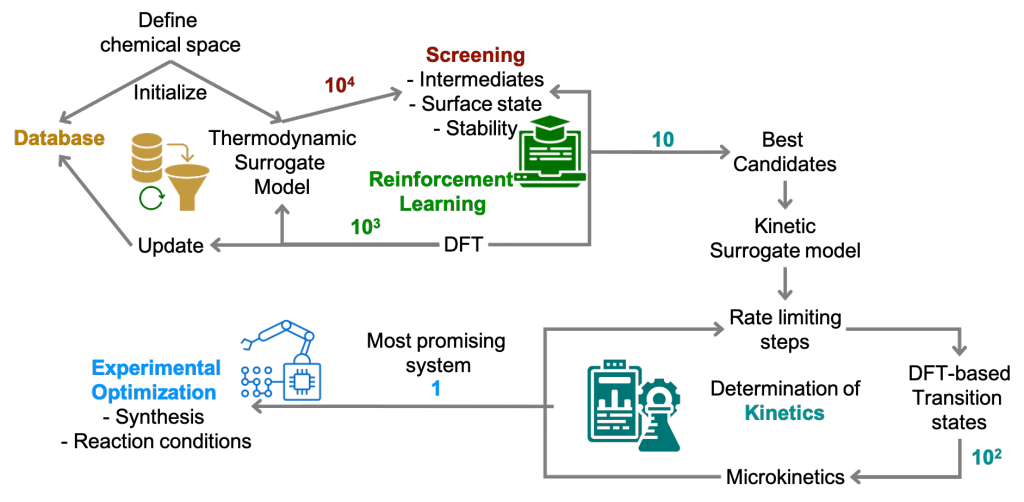
After some tedious work on a somewhat ill-defined problem, we are happy to announce the publication of our manuscript on the stability of 2H-MoS2 edges during the electrocatalytic hydrogen evolution reaction in acidic media in J. Chem. Phys. C. The main issue is how to determine the stability of a sulfided edge in the absence of a well-defined sulfur chemical potential? – Indeed, in contrast to typical catalytic applications of MoS2, there is simply no sulfur reservoir under HER and, thus, each S that is released in the form of H2S is irreversibly lost. Our investigation concludes that the Mo-edge is likely to exchange its surface sulfur by surface OH*. These sites remain active for HER. The S-edge, on the other hand, is expected to be more stable, but once it starts loosing its S atoms, the HER activity will drop, leading to deactivation phenomena.
TOC_ex