It is my great pleasure to announce the publication of the grand-canonical framework applied to the random phase approximation. Ziyang Wei from the group of Philippe Sautet did a terrific job in putting everything together and demonstrating the usefulness of this approach. Just check-out the manuscript in J. Phys. Chem. Letters.
Best Poster Prize
Congratulations to Nawras Abidi who won the best poster prize at the ISE Topical Meeting in Aachen. Well done and well deserved!
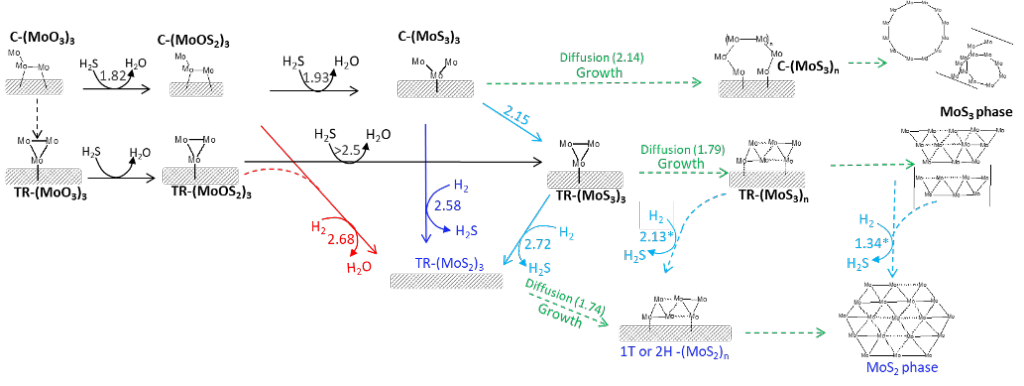
Genesis of MoS2 from model-Mo-oxide precursors supported on γ-alumina
After a very long reviewing process (with in the end only minor changes), I am very pleased to announce that the second article of Amit Sahu is finally published! If you are interested in the genesis of alumina supported MoS2 catalysts, check out the extensive computational work!
Autonomous high-throughput computations in catalysis
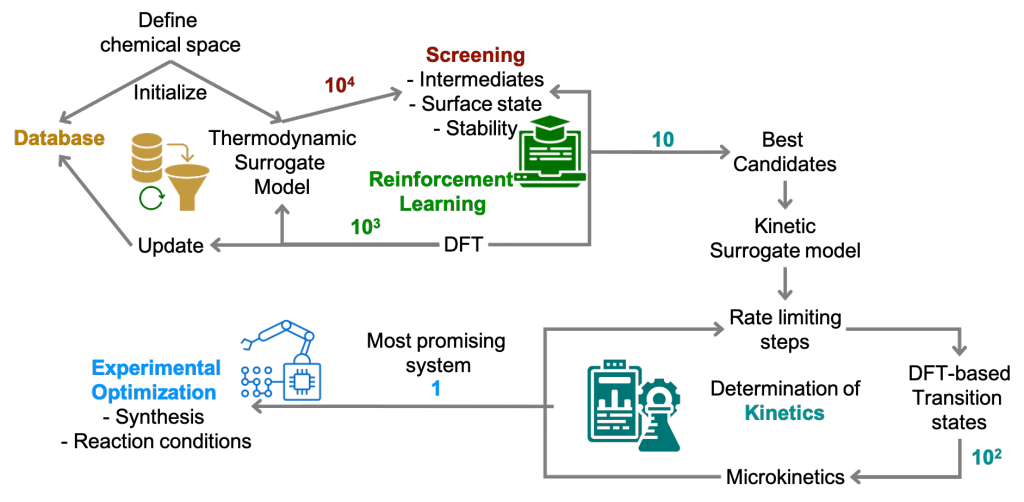
After quite some effort, the perspective on hight-throughput computations is finally online. Working with Zhi Wei Seh is always productive and Mohammed did a good job summarising the literature!
Review on Transition States in Electrocatalysis
Our first article of 2022 is now available in Current Opinion in Electrochemistry: We discuss the atomistic modelling of the identification of (potential-dependent) activation energies in electrocatalysis. Nawras did a great job!
TOC_eTSAttractive Gaussian Potentials for Force Fields Describing the Oxide/Organic Molecules Interaction
After some battling, we are very happy to present our work on interfacial force fields, now published in J. Phys. Chem. B: for near-chemisorption, as observed for Lewis-acid/Lewis-base interactions on metal oxides, the “standard” Lennard-Jones interactions should be supplemented with an attractive, anisotropic Gaussian attractive term in order to retrieve qualitatively correct results.
Stability of MoS2 Edges During HER
After some tedious work on a somewhat ill-defined problem, we are happy to announce the publication of our manuscript on the stability of 2H-MoS2 edges during the electrocatalytic hydrogen evolution reaction in acidic media in J. Chem. Phys. C. The main issue is how to determine the stability of a sulfided edge in the absence of a well-defined sulfur chemical potential? – Indeed, in contrast to typical catalytic applications of MoS2, there is simply no sulfur reservoir under HER and, thus, each S that is released in the form of H2S is irreversibly lost. Our investigation concludes that the Mo-edge is likely to exchange its surface sulfur by surface OH*. These sites remain active for HER. The S-edge, on the other hand, is expected to be more stable, but once it starts loosing its S atoms, the HER activity will drop, leading to deactivation phenomena.
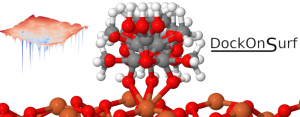
TOC_exDockOnSurf
We are very proud to announce that our paper describing the DockOnSurf package is now published in Journal of Chemical Information and Modeling.
The code is available on gitlab! And you can find its documentation on readthedocs.
If you have molecules to adsorb on a surface, this package is for you! – At least if you are using VASP or CP2K.
Designing Active Sites for Structure-Sensitive Reactions
Our very collaborative work on using generalized coordination numbers for understanding and designing active sites for alcohol dehydrogenation reactions has finally been published in J. Phys. Chem. C.
Understanding electrified interfaces
The comment I co-authored with Zhi Wei Seh from A*STAR in Nature Reviews Materials is now online! – We highlight the challenges and necessities for a better characterization and understanding of electrified interfaces. This is an important step towards a more rational design of electrocatalysts.
NatRevMatter