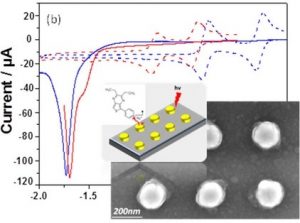
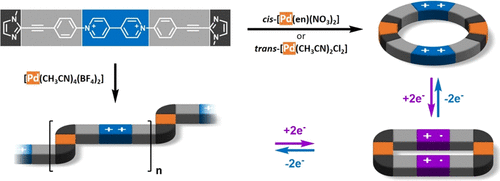
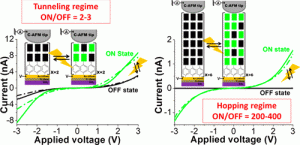
Our article is available to read at J. Am. Chem. Soc.
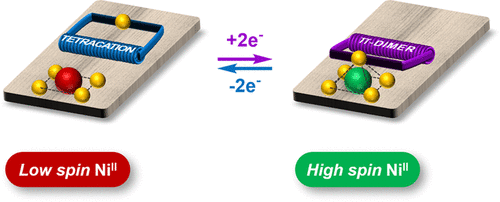 We herein report the synthesis and magnetic properties of a Ni(II)-porphyrin tethered to an imidazole ligand through a flexible electron-responsive mechanical hinge. The latter is capable of undergoing a large amplitude and fully reversible folding motion under the effect of electrical stimulation. This redox-triggered movement is exploited to force the axial coordination of the appended imidazole ligand onto the square-planar Ni(II) center, resulting in a change in its spin state from low spin (S = 0) to high spin (S = 1) proceeding with an 80% switching efficiency. The driving force of this reversible folding motion is the π-dimerization between two electrogenerated viologen cation radicals. The folding motion and the associated spin state switching are demonstrated on the grounds of NMR, (spectro)electrochemical, and magnetic data supported by quantum calculations.
We herein report the synthesis and magnetic properties of a Ni(II)-porphyrin tethered to an imidazole ligand through a flexible electron-responsive mechanical hinge. The latter is capable of undergoing a large amplitude and fully reversible folding motion under the effect of electrical stimulation. This redox-triggered movement is exploited to force the axial coordination of the appended imidazole ligand onto the square-planar Ni(II) center, resulting in a change in its spin state from low spin (S = 0) to high spin (S = 1) proceeding with an 80% switching efficiency. The driving force of this reversible folding motion is the π-dimerization between two electrogenerated viologen cation radicals. The folding motion and the associated spin state switching are demonstrated on the grounds of NMR, (spectro)electrochemical, and magnetic data supported by quantum calculations.