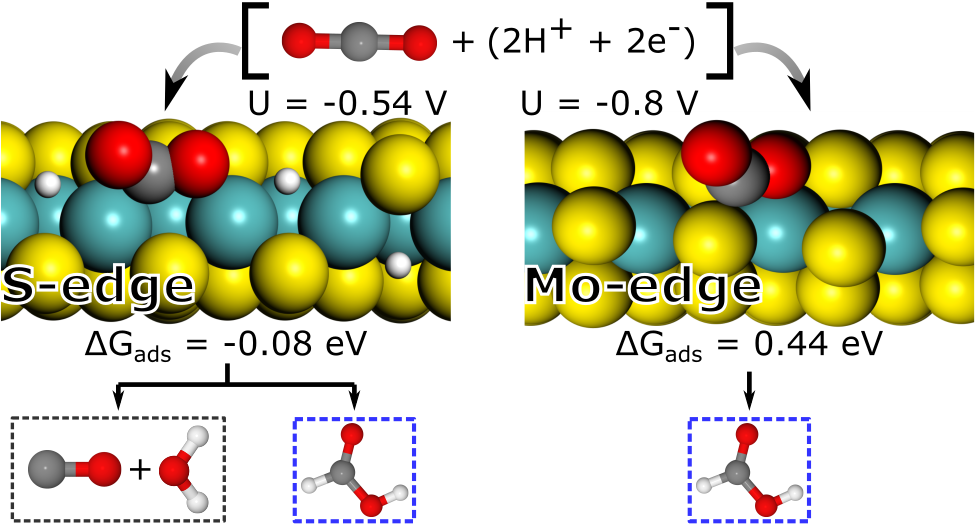
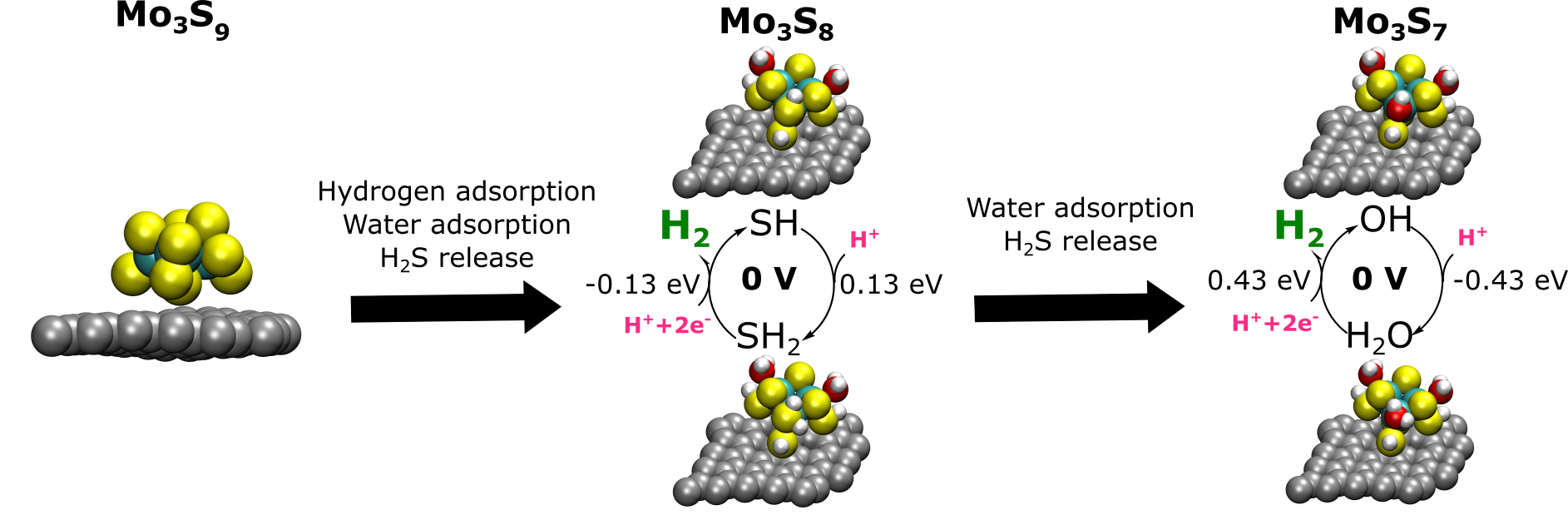
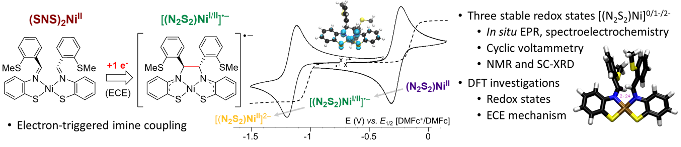
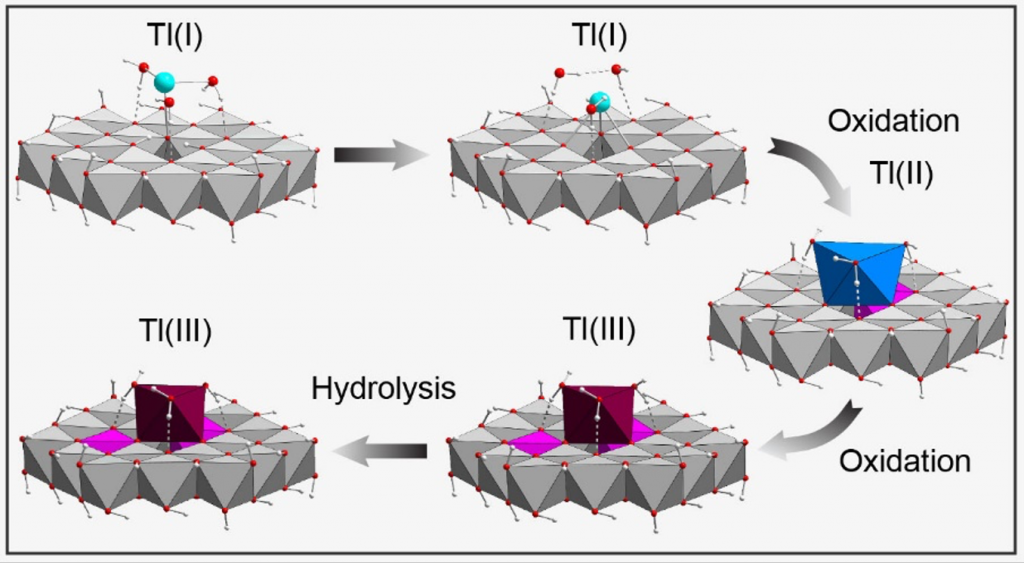
Akif has done amazing work in finalising a compelling manuscript on the use of MoS2 as a (photo)electro-cocatalysis for carbon dioxide reduction: The hydrogen coverage is intermediate to low under CO2 reduction potentials and the Mo- and S-edge have quite different reactivities, including for the CO2 adsorption. If you are interested in the reduction to CO and/or HCOOH, have a look at our J. Phys. Chem. C. paper