Research
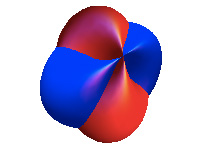
I study solids at the atomic level. Solids are made of atoms located at very specific spots in space, between which electrons move around and interact. The way the latter happens (the electrons behave) governs much of the electronic and magnetic properties of solids at a macroscopic level, that is, how we can observe them every day.
Magnetism
For example, why those magnets stick on your fridge can be explained at the electronic level. Indeed, you may know that electrons are "charged", so that they will interact with other charged objects (e.g. other electrons), and also create a "current" when they move in a coordinated fashion. But this is not all. They also carry "spin". Spin is a much more complex property than charge. Indeed, while charge is a fixed number (1.6 10^-19 Coulomb for the electron), spin is a vector (i.e. arrow) of fixed length. In particular, the spin (the arrow of fixed length) is free to point in any direction.
Frustrated Magnetism
The existence of the spin, and how to work with it (which involves quantum mechanics), has been known for a long time. Nowadays, the job of physicists in magnetism is try to understand how the electrons and ions interact with one another through their spins (~ where the arrows like to point), when there are so many of them in a solid, and what the resulting macroscopic properties are. Sometimes, things are very simple. Typically, things are not simple, and people then refer to "frustrated magnetism". To be more precise, one needs to know that objects try to get organized in such a way so as to minimize their energy. But it is not always possible to satisfy every one of them. That is when a system is said to be "frustrated", in close analogy with how a living being may feel.
Quantum Spin Liquids
It turns out that "frustrated" systems can often "choose" between different states (way they are organized) that have exactly the same energy. When this happens, the system sometimes tends to go from one state to another, or to be in all of these states at once (as allowed by quantum mechanics). The resulting properties are then truly exotic. For example, "quantum spin liquids" are states which look disorganized (like molecules that move around in a liquid) but actually give way to "emergent fractional particles". The latter are, for example, particles with the spin magnitude of the electron but no charge...and while this may not sound any more exotic than all of the above ;) , it is for physicists, since such particles can only emerge from a clever mixture of quantum physics.
La brouette ou les grandes inventions
Le paon fait la rouele hasard fait le reste
Dieu s'assoie dedans
et l'homme le pousse.
Jacques Prévert, Paroles
Publications
-
 Singular Angular Magnetoresistance
Singular Angular Magnetoresistance -
 Twisted bilayer graphene
Twisted bilayer graphene -
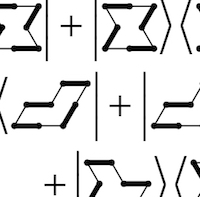 SU(4) spins and dimers
SU(4) spins and dimers -
 Quantization of the thermal Hall conductivity at small Hall angles
Quantization of the thermal Hall conductivity at small Hall angles -
 Loop States in Spin-Orbital Models on the Honeycomb Lattice
Loop States in Spin-Orbital Models on the Honeycomb Lattice -
 Superconductivity in Spin-Orbit Coupled Semimetals
Superconductivity in Spin-Orbit Coupled Semimetals -
 Probing Hidden Orders with Resonant Inelastic X-Ray Scattering
Probing Hidden Orders with Resonant Inelastic X-Ray Scattering -
 A New Type of Quantum Criticality in the Pyrochlore Iridates
A New Type of Quantum Criticality in the Pyrochlore Iridates -
 Definitive Evidence for Order-by-Quantum-Disorder in Er2Ti2O7
Definitive Evidence for Order-by-Quantum-Disorder in Er2Ti2O7 -
 Quantum Spin Liquid in Quantum Spin Ice
Quantum Spin Liquid in Quantum Spin Ice -
 Impurity Effects in Highly Frustrated Diamond Lattice Antiferromagnets
Impurity Effects in Highly Frustrated Diamond Lattice Antiferromagnets -
 Fractional Vortices on Twin-Boundaries in Non-Centrosymmetric Superconductors
Fractional Vortices on Twin-Boundaries in Non-Centrosymmetric Superconductors
 Lucile Savary
Lucile Savary