Modelling the S-Doped Sodalites Using DFT, TD-DFT and SAC-CI Methods
A. Curutchet and T. Le Bahers, Inorg. Chem. 2017, 56, 414-423
It corresponds to my first published work in the field of tenebrescent materials. It is a purely computational work aiming to demonstrate that quantum chemistry can be used to model the photochromism in sodalites.
Among the most important results, we show that to model the absorption spectrum of the F-center by a cluster approach, the full β-cage must be considered in the quantum model along with the vibronic-coupling. The electronic transition of the trapped electron strongly couples with the breathing mode of the Na4 tetrahedron.
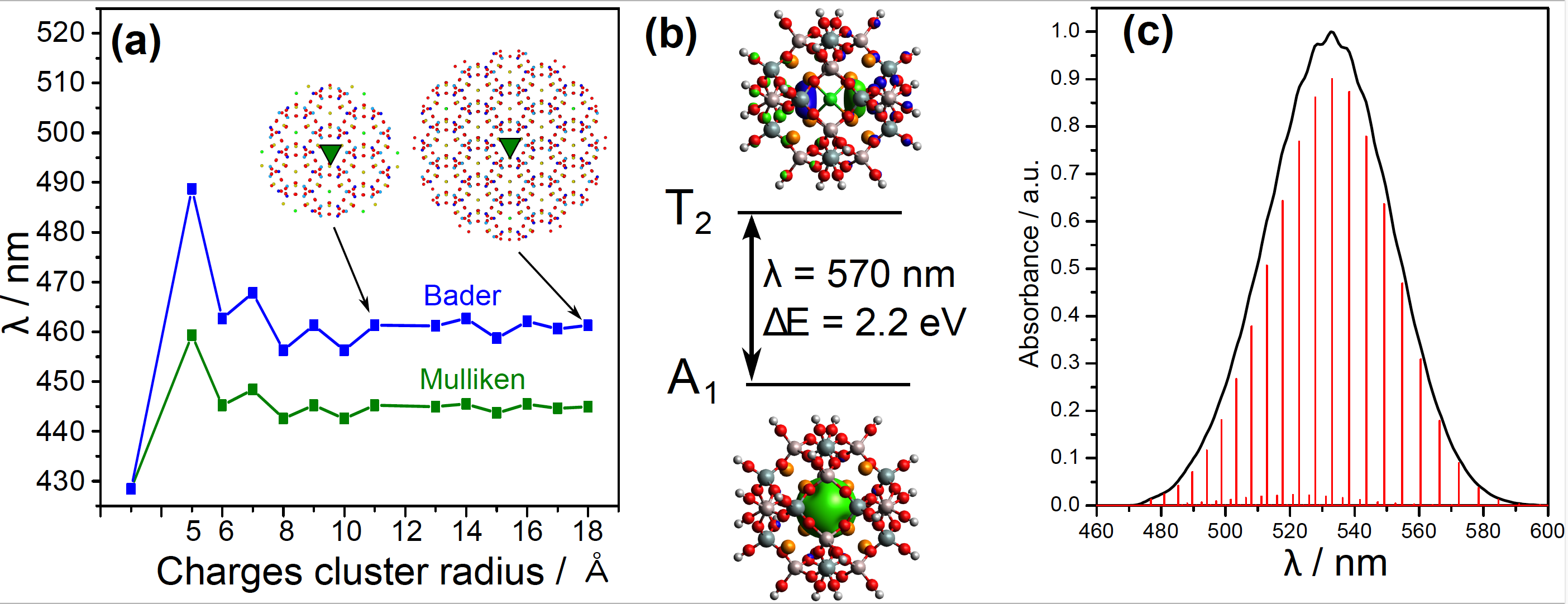
Figure 1: (a) TD-DFT absorption wavelength of the [Na4VCl]3+ system surrounded by a cluster of point charge (Mulliken or Bader charges) of increasing size. In inset, the green triangle is the quantum part and the points are the charge positions. (b) TD-DFT computed absorption wavelength of a cluster including β-cage, without point charges. (c) Simulated vibronic coupling of the absorption, including only the vibration of the Na4 tetrahedron.
We prove that the charge transfer from the S22- impurity to the chlorine vacancy is responsible of the F-center formation with transition energies in agreement with the experiment.
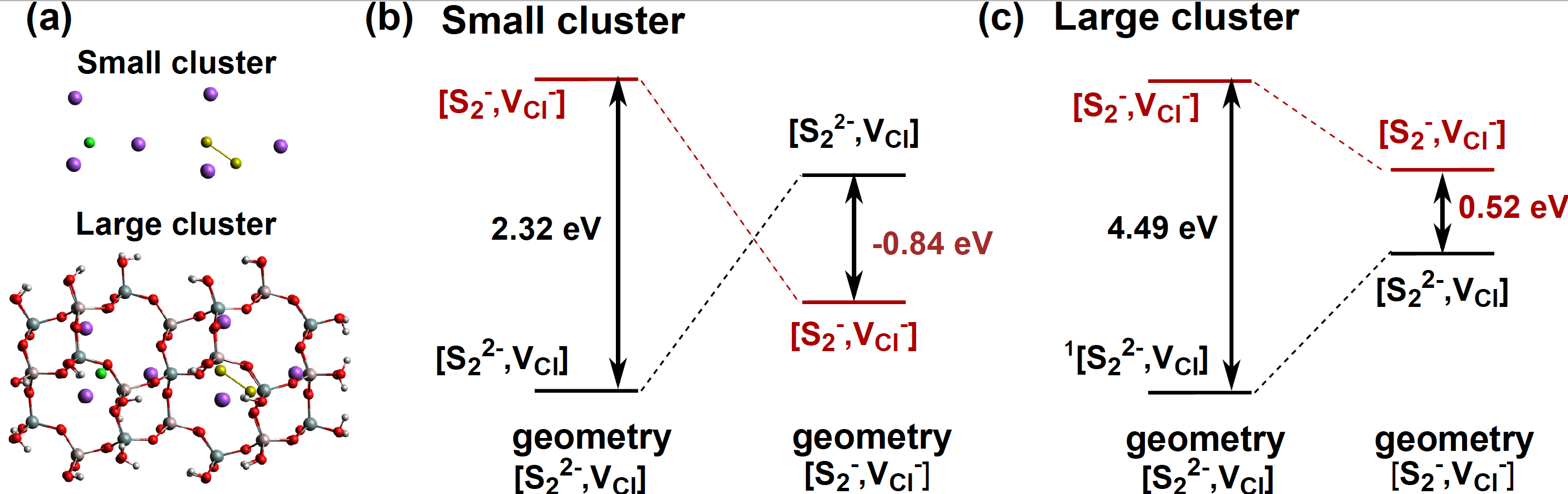
Figure 2: (a) Structure of the small and large clusters. (b) and (c) SAC-CI transition energies computed for the two clusters for two geometries.
Solar UV Index and UV Dose Determination with Photochromic Hackmanites: From the Assessment of Fundamental Properties to the Device
I. Norrbo, A. Curutchet, A. Kuusisto, J. Mäkelä, P. Laukannen, P. Paturi, T. Laihinen, J. Sinkkonen, E. Wetterskog, F. Mamedov, T. Le Bahers, M. Lastusaari, Mater. Horiz. 2018, 5, 569-576
This article is the first of the collaboration with the group of Mika Lastusaari. In this work, we proved both experimentally and computationally that a partial replacement of Na by other larger alkaline atoms (K and Rb) leads to a lowering of the transition energy of the electron transfer from S22- to the chlorine vacancy.
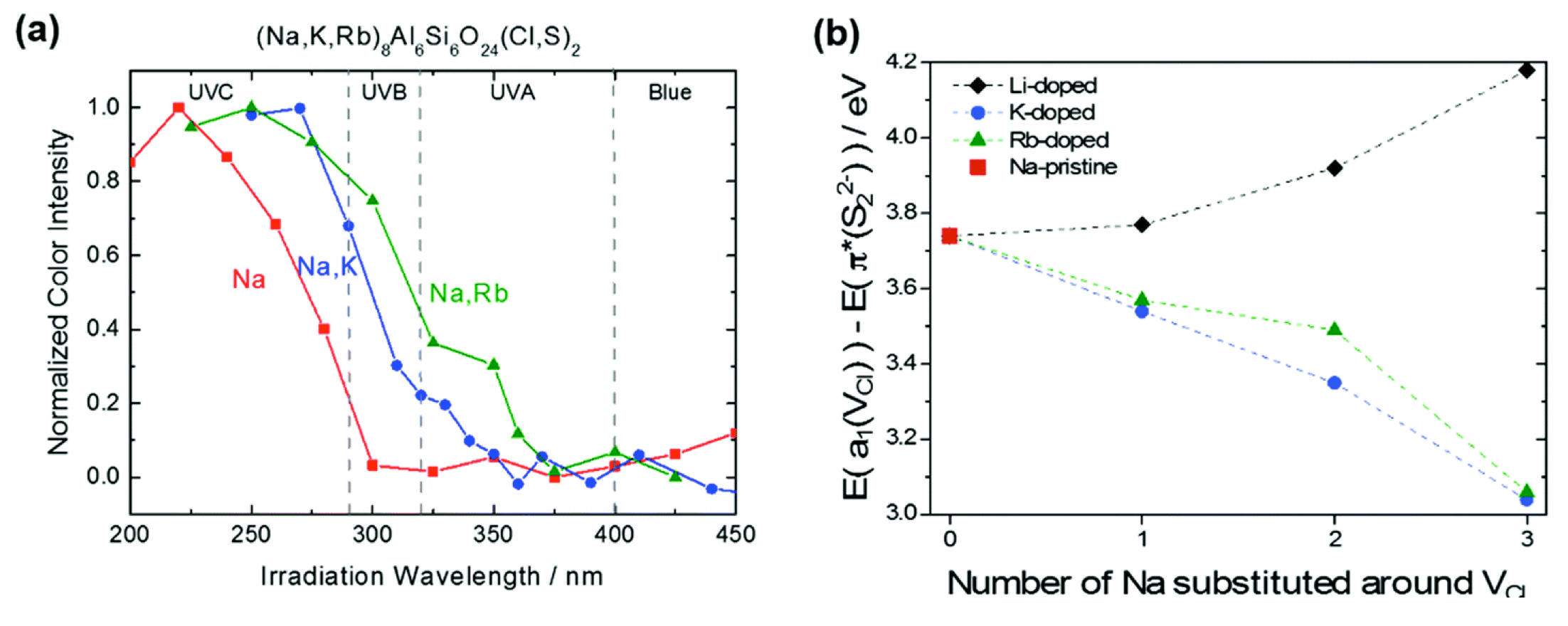
Figure 3: (a) Tenebrescence excitation spectra of (Na,M)8Al6Si6O24(Cl,S)2 (M = none, K and Rb) with 6% of S doping. (b) DFT computed evolution of the energy gap (in eV) between the last occupied orbital π*(S22−) and the first unoccupied orbital a1(VCl) as a function of the number of Na substituted around the Cl vacancy.
Furthermore, in this work the group of Mika Lastusaari presents for the first time the thermotenebrescence experiment allowing to measure the activation energy for the bleaching of the material. Interestingly, the activation energy measured (around 0.4 eV) is in very good agreement with the one proposed by DFT calculation in our previous article.
Finally, we propose to use artificial tenebrescent hackmanites as UV indicator because the sensitivity of the material toward UV light can be tuned and adapted for specific UV light (such as UV from the sunlight).
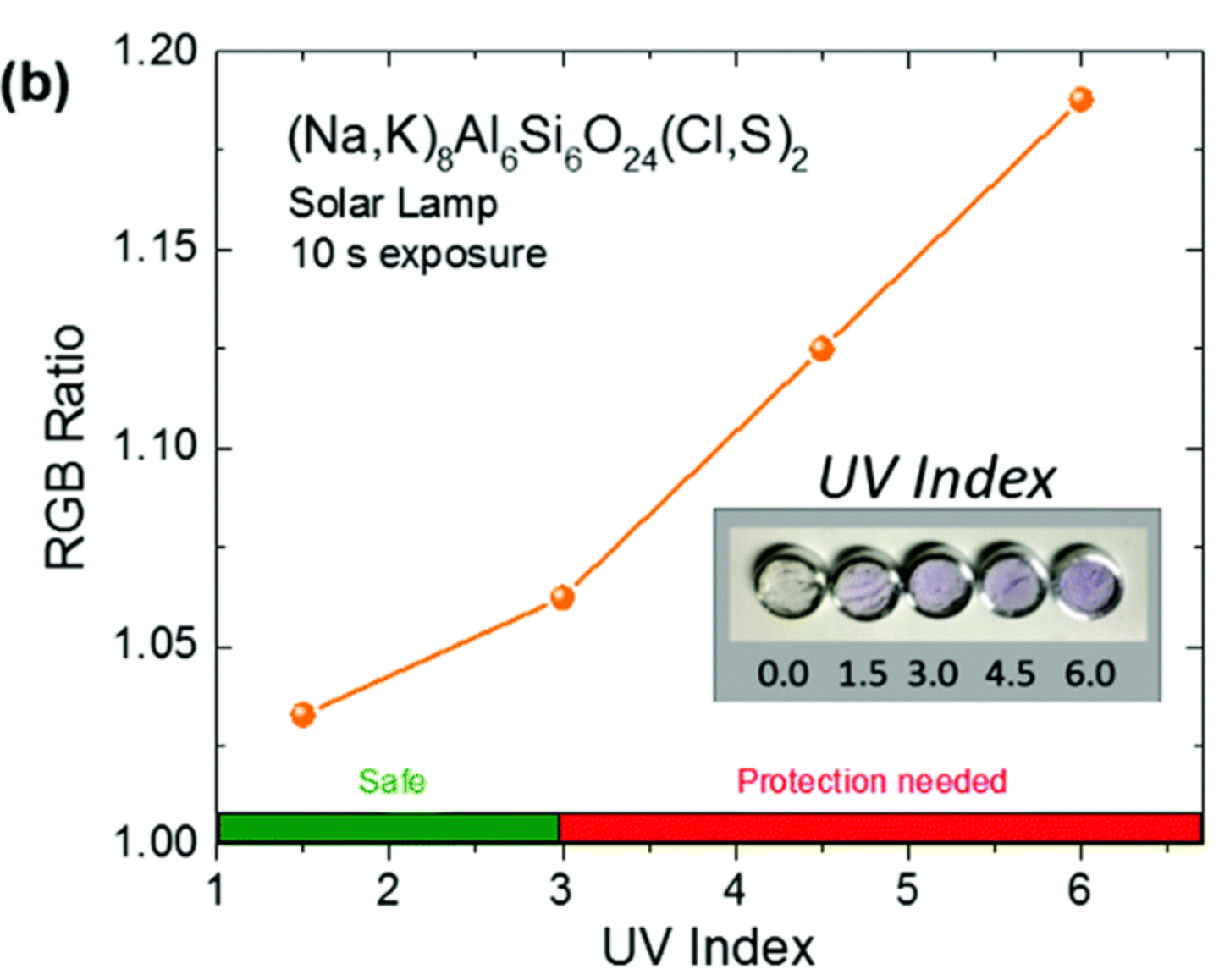
Figure 4: Color intensity of (Na,K)8Al6Si6O24(Cl,S)2 at different UV index values.
On the Spectroscopic Modeling of Localized Defects in Sodalites by TD-DFT
P. Colinet, A. Gheeraert, A. Curutchet, and T. Le Bahers, J. Phys. Chem. C, 2020, XX, XX-XX
In this study we refined the cluster approach introduced in my first article on tenebrescent minerals, and investigate two different type of localized defects in sodalite: trapped electrons or dichalcogen anions.
Figure 1: Absorption of the trapped electron with its orbitals delocalized on the cage (left); emission of the disulfur with its orbitals localized onto the defect (right).
Figure 2: Simulated F-center absorption (left) and S2- emission (right) spectra with different embedding. TD-PBE0 results convoluted by a Gaussian are represented by the red dotted line. The blue curves are obtained by the addition of vibronic coupling.
One of the main conclusions concerns the influence of the close environment for the spectroscopic properties. We show that a sophisticated embedding is necessary in the case of delocalization of the orbitals at stake on the β-cage. Hence, a quick analysis of the delocalization of the defect orbitals seems to be a good indicator of the necessity to include an embedding around the QM cluster.
Hackmanite - The Natural Glow in the Dark Mineral
C. Agamah, S. Vuori, P. Colinet, I. Norrbo, J. Miranda de Cavalho, N. K. O. Nakamura, J. Lindblom, L. van Goethem, A. Emmermann, T. Saarinen, T. Laihinen, E. Laakkonen, J. Lindén, K. Konu, H. Vrielinck, D. Van der Haagen, P. F. Smet, T. Le Bahers, M. Lastusaari, Chem. Mater. 2020, 32, 8895-8905.
This article presents another spectroscopic phenomenon observed in hackmanites, the persistent luminescence. We have shown that this luminescence persistent is due to Ti3+ ions which, upon light excitation, allows the transfer of an electron from Ti3+ to an oxygen vacancy. This is the thermally activated return of the electron to the titane that is responsible of the luminescence. We have also showwn that there is a competition between the luminescence phenomenon and the photochromism. The areas where the photochromisme is observed do exhibit any persistent luminescence. By combining DFT and TD-dfT calculations with experiments, we have assumed that the photons emitted by the persistent luminescence are absorbed by the charge transfer transition caracteristic of photochromism.
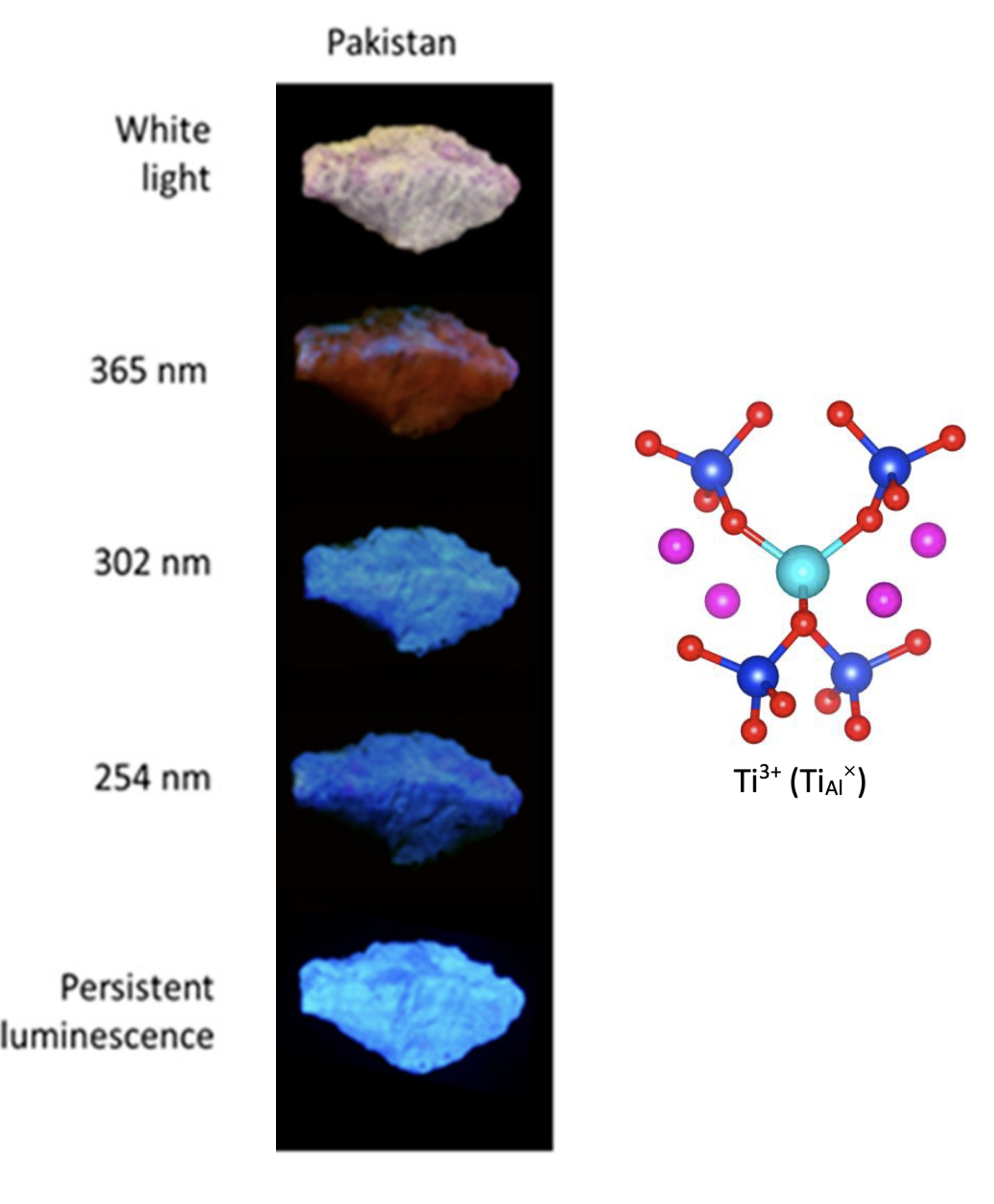
Figure 7: Photo of the hackmanite's emission as a funtion of the excitation wavelenght (left). Cluster used to simulate the spesctrocpique properties of Ti3+ (right).
Detection of X-ray Doses with Color-Changing Hackmanites: Mechanism and Application
S. Vuori, P. Colinet, I. Norrbo, R. Steininger, T. Saarinen, H. Palonen, P. Paturi, L. C. V. Rodrigues, J. Göttlincher, T. Le Bahers, M. Lastusaari, Adv. Opt. Mater., 2021, 9, 2100762.
In this work, wwe have shown that the photochromism can also be observed in hackmanites exposed to X-ray. However, the mecancism is different from the ones involving UV light. For X-ray, this is not a charge transfer transition that is responsible of the F-center, but a scintillation mechanism. X-rays generate a large number of "hot" electrons and holes in the material. The excited electrons populate the chlorine vacancy giving rise to the F-centers.
We have shown that it is possible to use an hackmanite film as an X-ray detecteur or to do imagery (Figure 8).
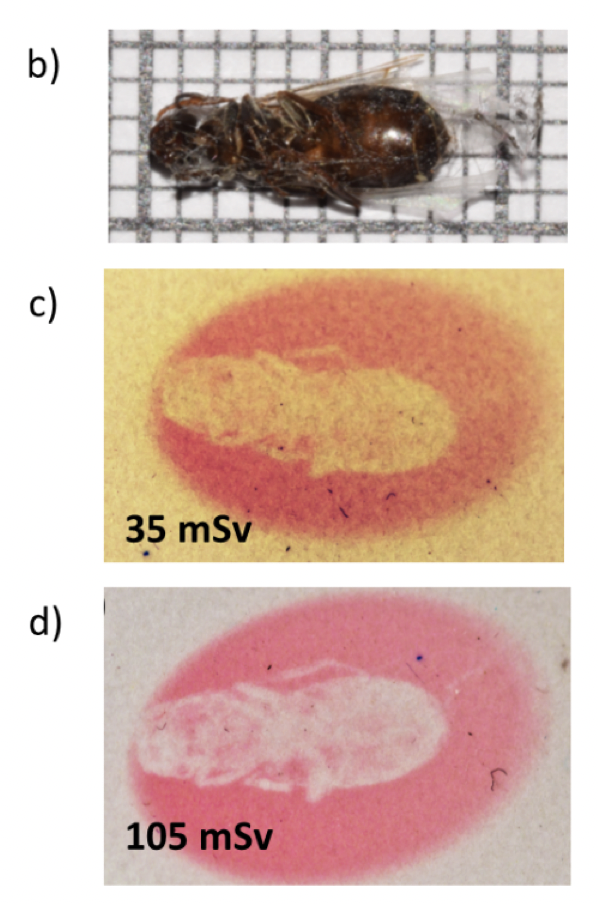
Figure 8: Photo of a fly exposed to different X-ray dose.


