The photochromism
The photochromism consists of a reversible change of colour of a compound under light exposure, mainly in the UV part of the spectrum.
This phenomenon can find several technical applications mainly for adaptive glasses (such as self-adaptive sunglasses), optical memories and molecular switches. Nowadays, the effort to develop new photochromic materials focuses on molecular materials made of organic molecules. The diarylethen (Figure 1) and azobenzen families are the most investigated molecular families. Under UV exposure, these molecules can experience chemical bond formations or intra-molecular reorientations responsible of the photochromic activity. The attractiveness of organic molecules comes from the ability of the organic chemistry to tune and design molecules to adapt their properties to the wanted applications. 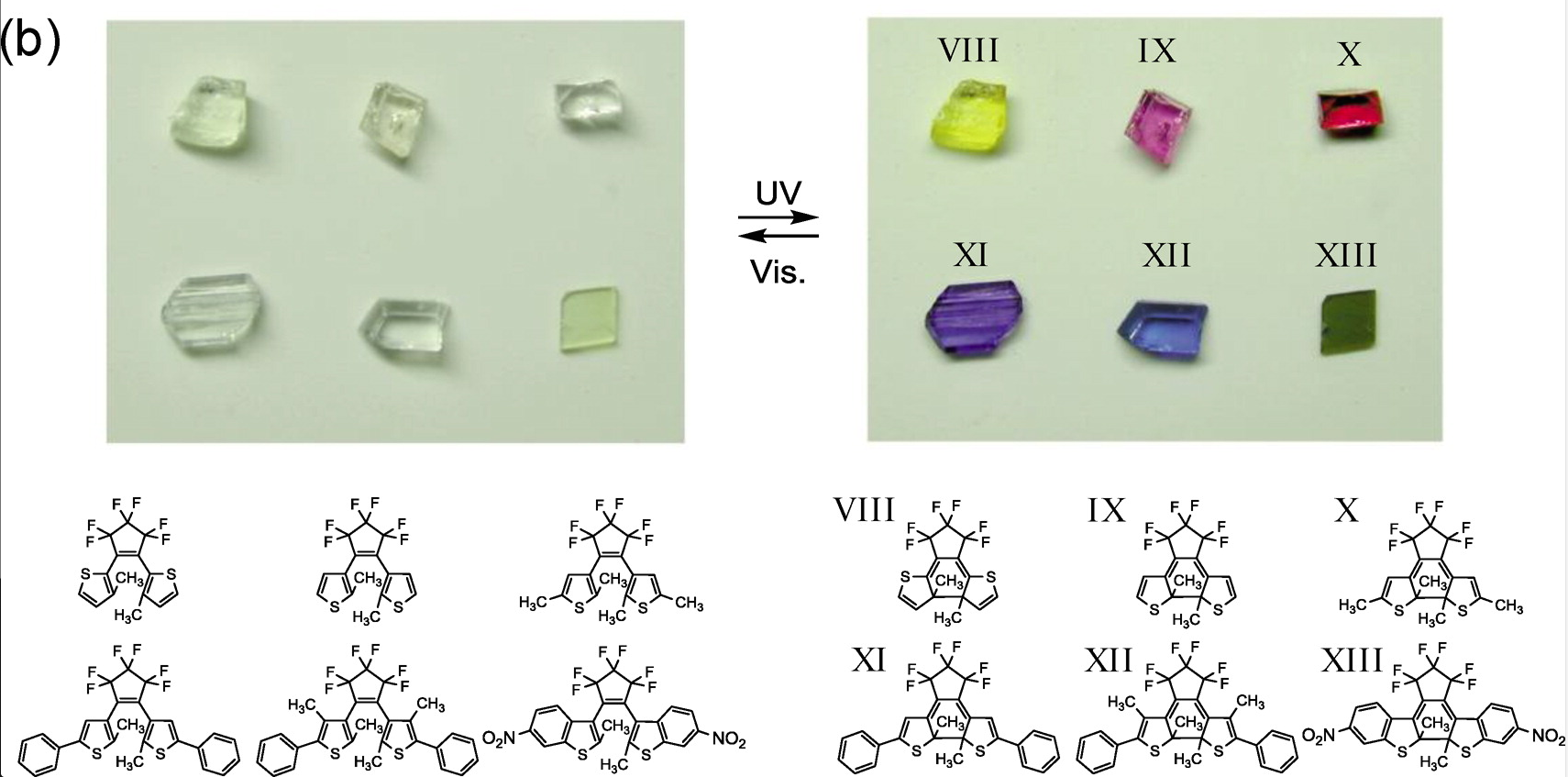
Figure 1 : Diarylethens based molecular materials. (Reprinted with permission from (Irie et al. Chem. Rev. 2014, 114, 12174). Copyright (2017) American Chemical Society)1.
However, a community tries to develop photochromic systems made of inorganic materials, typically tungsten (WO3) and molybdenum (MoO3) oxides. The drawback of these inorganic materials is their lack of adaptability compared to organic materials even if they have several interesting properties such as a good thermal stability and a good stability against moisture.
The tenebrescence
The tenebrescence is the name given in geology for photochromism.
Far from the researches on new photochromic materials for high-tech applications stand natural tenebrescent minerals, known only by geologists and some well-documented persons. From an historical point of view, the tenebrescence of natural minerals was first reported in the beginning the 20th century.2,3
More specifically, the photochromism of some sodalites, with the Na8(SiAlO6)Cl2 formula, was investigated deeply since the middle of the 20th century and during the 70’s-80’s. 4–12 The tenebrescence of these sodalites is characterized by the colouring from a white to a purple form of the mineral after an exposure to a 250-275 nm (4.5-5 eV) irradiation. Quickly, it appeared that the mechanism of the phenomenon involved sulfur-based impurities, probably the S22- ion.
To well understand the mechanism of the photochromism proposed in the literature, the crystal structure of the sodalite must be known. The sodalite crystalize in the cubic structure with the P-43n space group. The Si, Al and O atoms form a structure called β-cage. A tetrahedron of Na+ ion is placed inside this cage while a Cl- ion stands in the middle of the tetrahedron (Figure 2).
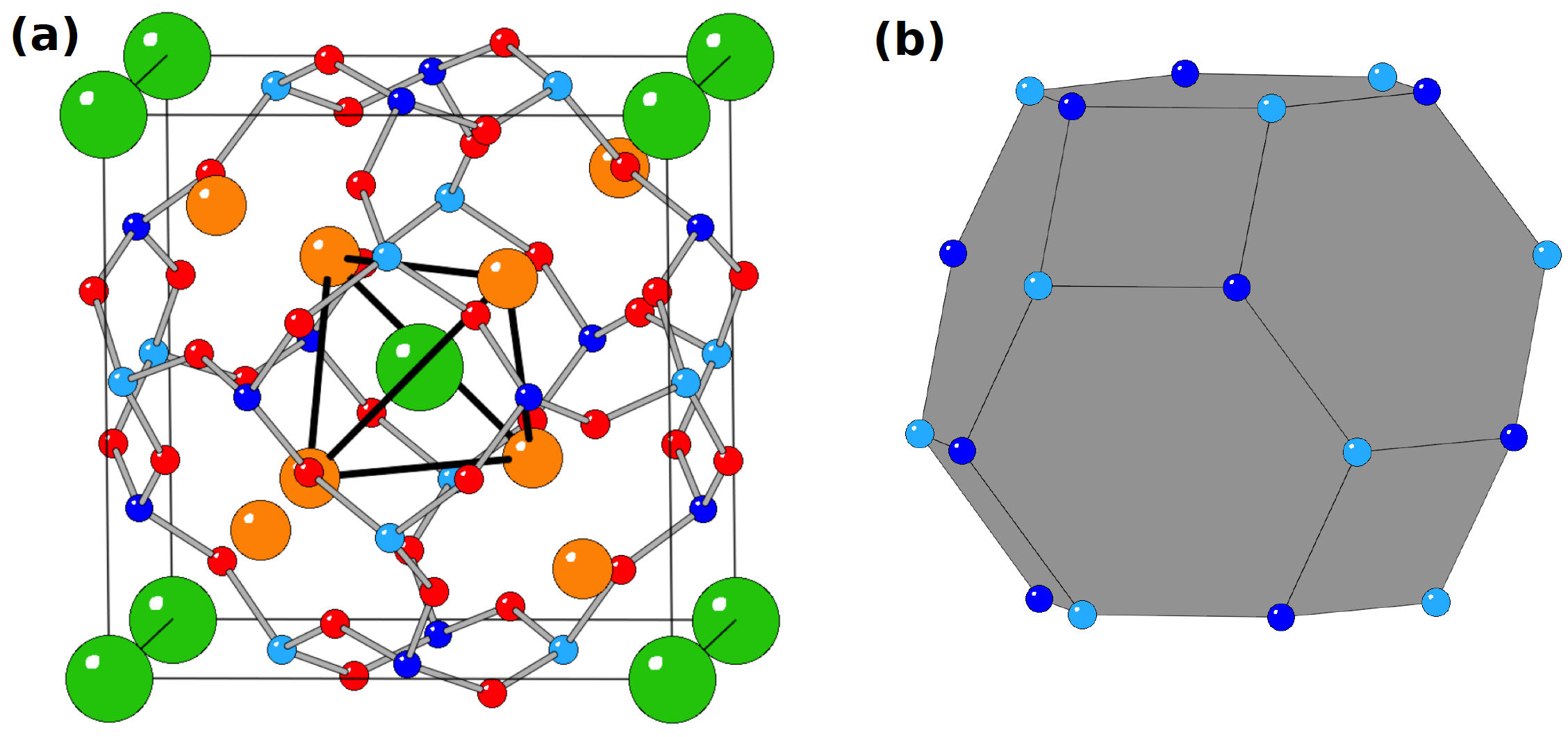
Figure 2: (a) Sodalite cell. The Si, Al, Na, Cl and O atoms are in blue, cyan, orange, green and red respectively. The Si-O and Al-O bonds are depicted by grey lines and the edges of the Na+ tetrahedron are presented by black lines. Reprinted with permission from (A. Curutchet and T. Le Bahers, Inorg. Chem. 2017, 56, 414-423). Copyright (2017) American Chemical Society (b) Scheme of the β-cage.
The mechanism proposed for the photochromism is the following one 13 (Figure 3):
(1) The S22- ion substitutes a Cl- ion in the crystal, leading the formation of a Cl- vacancy to keep the electroneutrality of the crystal.
(2) Under UV irradiation, the S22- ion transfers an electron toward the vacancy, VCl.
(3) An electron trapped in a vacancy has quantified energy levels, leading to absorption in the visible part of the spectrum giving the colour.
(4) Under the influence of white light or heating, the trapped electron returns to the S2- ion, bleaching the material. 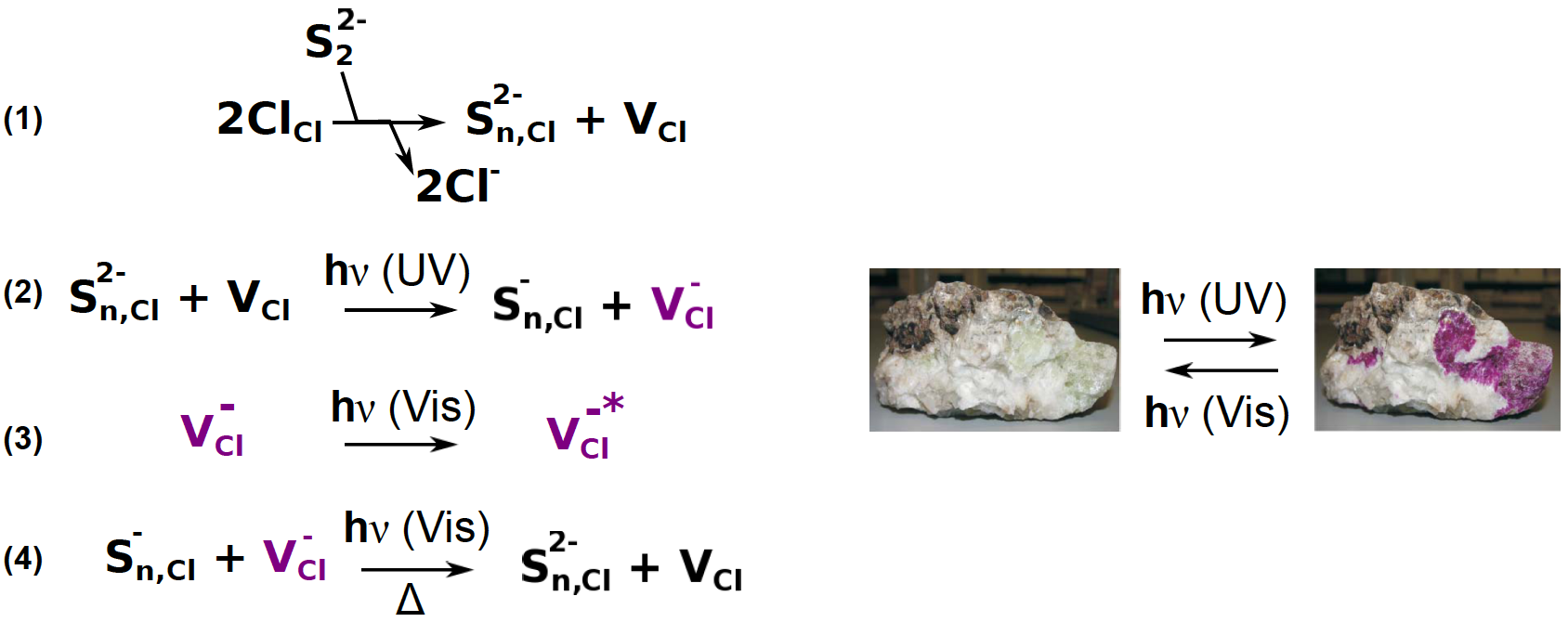
Figure 3: working principle of the sodalite tenebrescence. On the right, photos tenebrescent natural sodalite (adapted from ref [17] with permission from the Royal Society of Chemistry).
Because the spectroscopic properties of sulfur-doped sodalites are very different from the ones of the undoped sodalite, the tenebrescent sodalites are also called hackmanites.
The investigation of these minerals falls into disuse during some decades. But renewed of interest has appeared this decade due to the work of M. T. Weller and M. Lastusaari.14–17 They have shown that the hackmanite could be synthesized quite easily but also that the composition of the mineral could be tuned leading to a change of the colour of the coloured form. These works have opened the way to a design of hackmanites for photochromic materials with as many possibilities as organic materials.
From a fundamental point of view, other natural minerals are known for their tenebrescence such as scapolites and tugtupites.18 But these minerals have been less investigated than hackmanites. The mechanism expected for their tenebrescence is the same as hackmanites.
References
1 M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277.
2 E. W. Claffy, Am. Mineral., 1953, 38, 919.
3 S. C. Lind and D. C. Bardwell, J. Franklin Inst., 1923, 196, 375–390.
4 D. B. Medved, Am. Miner., 1954, 39, 615–629.
5 R. D. Kirk, Am. Miner., 1955, 40, 22–31.
6 H. D. Miser and J. J. Glass, Am. Mineral., 1941, 26, 437–445.
7 P. S. Pizani and M. C. Terrile, Am. Miner., 1985, 70, 1186–1192.
8 L. T. Todd, E. F. Farrell and A. Linz, IEEE Trans. Electron Devices, 1976, 23, 1183–1184.
9 V. P. Denks, A. E. Dudel’zak, C. B. Luschchik, T. V. Ruus, N. P. Soschchin and T. I. Trofimova, J. Appl. Spectrosc., 1976, 24, 23–28.
10 I. F. Chang and A. Onton, J. Electron. Mater., 1973, 2, 17–46.
11 D. W. G. Ballentyne and K. L. Bye, J. Phys. D Appl. Phys., 1970, 3, 1438–1443.
12 C. Z. Van Doorn, D. J. Schipper and P. T. Bolwijn, J. Electrochem. Soc., 1972, 119, 85–92.
13 A. Curutchet and T. Le Bahers, Inorg. Chem., 2017, 56, 414–423.
14 I. Norrbo, P. Gluchowski, I. Hyppänen, T. Laihinen, P. Laukkanen, J. Mäkelä, F. Mamedov, H. S. Santos, J. Sinkkonen, M. Tuomisto, A. Viinikanoja and M. Lastusaari, ACS Appl. Mater. Inter., 2016, 8, 11592–11602.
15 I. Norrbo, P. Gluchowski, P. Paturi, J. Sinkkonen and M. Lastusaari, Inorg. Chem., 2015, 54, 7717–7724.
16 E. R. Williams, A. Simmonds, J. A. Armstrong and M. T. Weller, J. Mater. Chem., 2010, 20, 10883–10887.
17 J. a Armstrong and M. T. Weller, Chem. Comm., 2006, 4, 1094–1096.
18 C. C. Milisenda, S. Koch, S. Müller, T. Stephan and M. Wild, Proceedings, IGC 2015, Vilnius, 2009, 107–109.


