Modelling the S-Doped Sodalites Using DFT, TD-DFT and SAC-CI Methods
A. Curutchet and T. Le Bahers, Inorg. Chem. 2017, 56, 414-423
It is our first publication on the topic of tenebrecsent minerals. This work, only based on quantum chemistry, aims at demonstrating that this tool can be used to model the photochromism in sodalites.
We demonstrated that for modelling the absorption spectra of a F-center using a cluster approach, a complete β-cage must be considered in the clustertreated at the quantum level and vibronic should be included. The electronic transition of the trapped electron is strongly coupled with the breathing mode of the sodium tetrahedron.
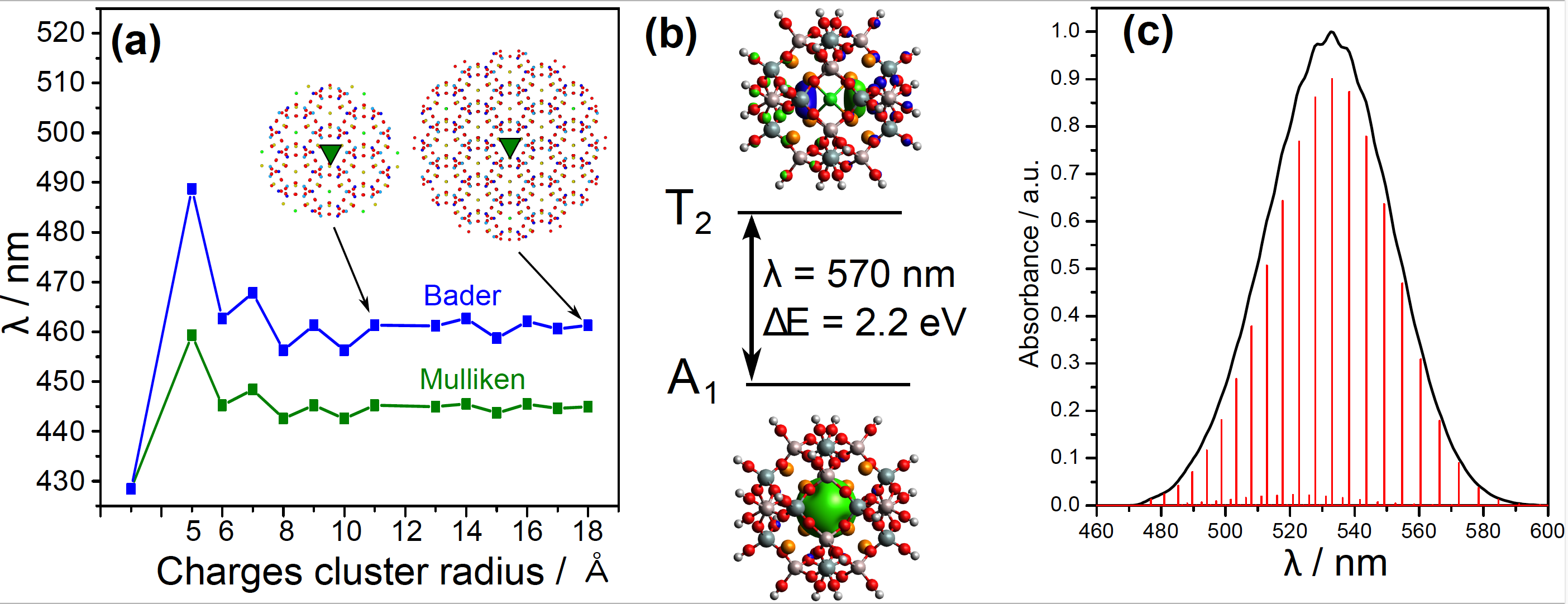
Figure 1: (a) Absorption wavelenght of the [Na4VCl]3+ system surrounded by an array of point charges (Bader and Mulliken) of increasing size. (b) Absorption wavelenght computed by TD-DFT of a cluster including a full β-cage without point charges.(c) Absorption spectra simulated by including the vibronic coupling of the Na4 tetrahedron.
We also demonstrated that the charge transfer from the S22- ion toward the chlorine vacancy is responsible of the trapped electron formation with transition energies in agreement with the experiment.
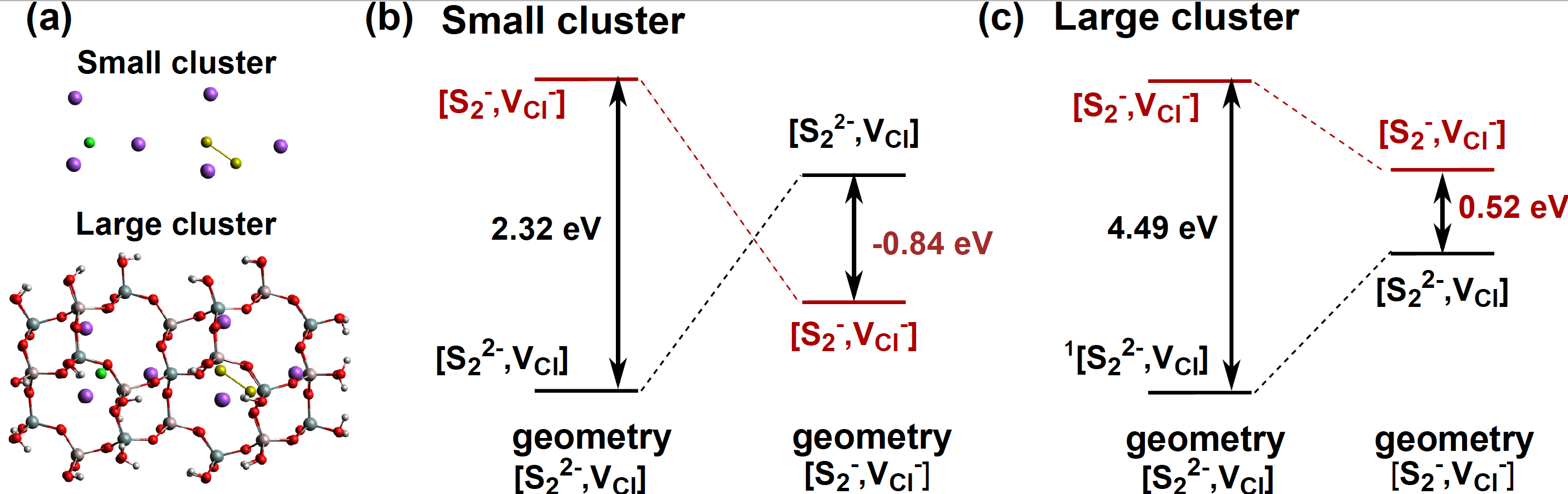
Figure 2: (a) Small and Large clusters structures (b) et (c) Transition energies computed at the SAC-CI level.
Solar UV Index and UV Dose Determination with Photochromic Hackmanites: From the Assessment of Fundamental Properties to the Device
I. Norrbo, A. Curutchet, A. Kuusisto, J. Mäkelä, P. Laukannen, P. Paturi, T. Laihinen, J. Sinkkonen, E. Wetterskog, F. Mamedov, T. Le Bahers, M. Lastusaari, Mater. Horiz. 2018, 5, 569-576
This article is first of the collaboration with the group of Mika Lastusaari. In this work, we proved experimentally and theoretically that the partial substitution of Na by other alkaline elements (such as K or Rb) induces a decrease of the transition energy associated to the charge transfer from S22- to the chlorine vacancy.
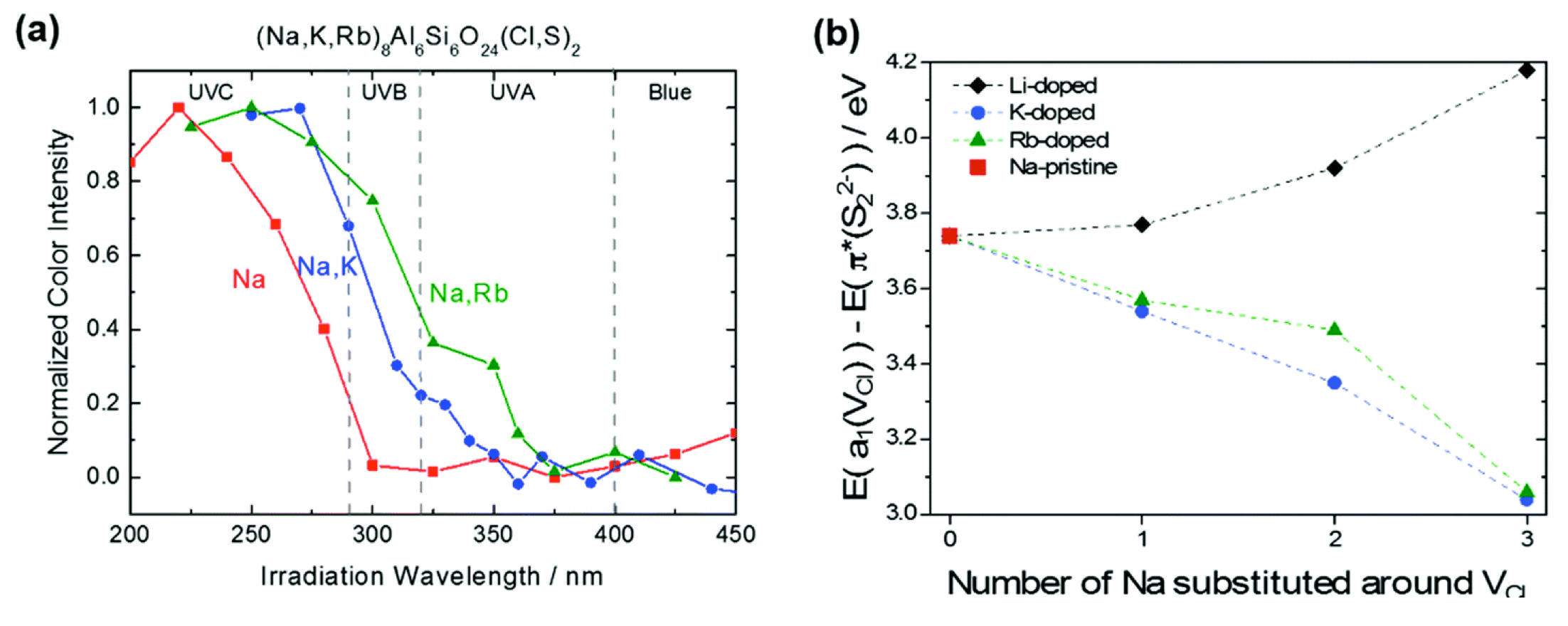
Figure 3: (a) Excitation spectra of the tenebrescence of (Na,M)8Al6Si6O24(Cl,S)2 (M = none, K et Rb) with 6% of S doping. (b) bandgap variation computed at the DFT level (en eV) between the last occupied orbital π*(S22−) and the first unoccupied orbital a1(VCl) as a function of the number of Na substituted aorund VCl.
Furthermore, in this work, the group of Mika Lastusaari presents for the first time the thermotenebrescence experiment allowing to measure the activation energy associated to the bleaching of the material. This measured activation energy (around 0.4 eV) is in nice agreement with the computed value published by us earlier.
Finally, we propose to use this artificial hackmanite as UV probe since the sensititivity to UV can be tuned to adapt to a specific UV target (such as UV from sun light).
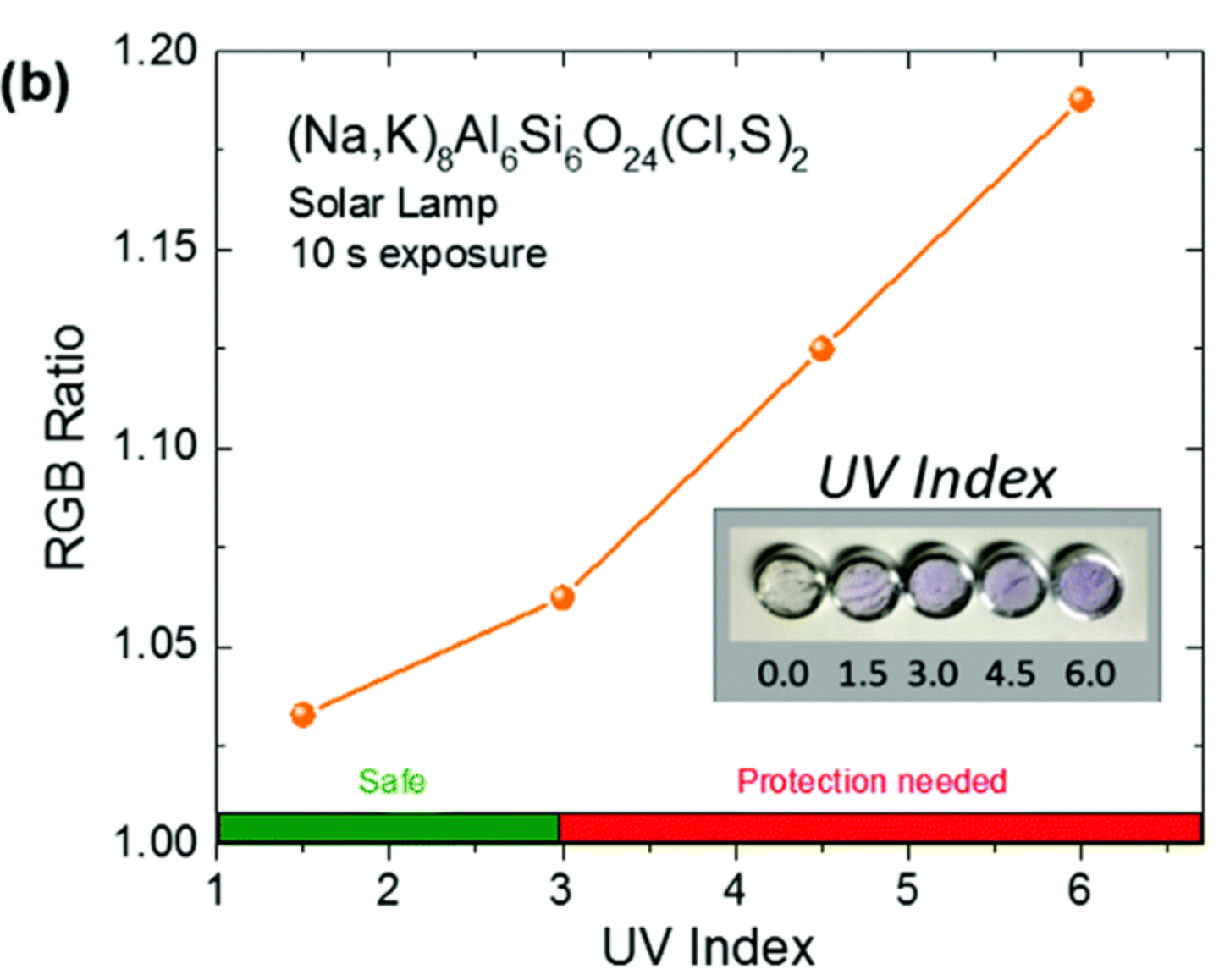
Figure 4: Color intensity of (Na,K)8Al6Si6O24(Cl,S)2 as a function of the UV index.
On the Spectrosocpic Modelling of Localized Defects in Sodalites by TD-DFT
P. Colinet, A. Gheeraert, A. Curutchet, T. Le Bahers, J. Phys. Chem. C, 2020, 124,
In this work, we refined the cluster approach introduced in our first article on tenebrescent minerals. This work focused on the simulation of the spectroscopic properties of two point defects: trapped electron and dichalcogen anions.
Figure 5: Orbitals involved in the absorption of the trapped electron (leeft) showing thee delocalization in the aluminosilicate cage. Orbitals invovled in the emission spectrum of S2- (right) showing no deelocalization on the aluminosilicate cage.
Figure 6: Absorption spectrum of F-center (left) and emission spectrum of the S2- ion (right) simulated for different embedding (TD-PBE0). The blue curve is obtained by considering the vibronic coupling.
The main conclusion is related to the influence of the embedding on the simulation of spectroscopic properties. While the F-center and the S2- ion are both point defect, they don't have the same sensitivity toward the embedding (Figure 6). A fast analysis of the delocalization of the orbitals (Figure 5) allows to understand if the embedding will play an important role or not.


